Did Sennelier Tinfix Silk Dyes cause my wife's hyperthyroidism?


It is very interesting that your Super Tinfix dye bottles bear a warning about possibly causing thyroid disease. How very disappointing that the Sennelier company refused to provide further information! I wonder if they are refusing to release this information in an attempt to avoid legal repercussions.
One acid dye that contains iodine is erythrosine, a food dye. Eating this dye in foods can result in the release of the iodine it contains within the body, which in large doses can certainly affect the thyroid. Although it is very possible that consuming large amounts of this dye, which is known as FD&C Red No. 3 or Colour Index Acid Red 51, would have effects on the thyroid, it seems unlikely that a dye which is used for food would be very harmful in the much smaller quantities that one might absorb as the result of accidental exposure while painting with it. Presumably only small quantities would have been spilled on the hands, and much smaller quantities absorbed orally, as the result of putting the hands to the mouth, eating, smoking, or anything else like that, before washing one's hands. It appears that a daily intake of FD&C red #3 of 60 milligrams daily long-term is without any effects in humans.
However, there certainly could be another acid red dye, besides erythrosine, which has thyroid effects. Food colors must pass animal feeding tests, but the other dyes which are used in products such as Super Tinfx dyes do not; if there are other acid dyes which contain iodine, their effects could, in theory, be much more significant than those of FD&C red #3. Unfortunately, I have not been able to locate the names of such dyes for you. There are hundreds of acid dyes whose Colour Index names begin with "Acid Red". There should be a number following that part of the name, but Sennelier omitted it as a part of their program of secrecy concerning the contents of their dyes. I have never been able to find out the exact identity of any of the French silk dyes, though I have been trying for years.
Whether the dyes themselves led to your wife's disease is open to question. It is not impossible, but autoimmune thyroid disease is common in developed countries, so it will be hard to be sure where to affix blame.
I believe that the most common cause of autoimmune thyroid disease, including Grave's disease and Hashimoto's thyroiditis, is excessive dietary intake of iodine, which is very widespread. In many countries, serious developmental defects, including mental retardation, arise as the result of iodine deficiency; in order to prevent this, many countries such as the US have fortified salt with iodine. The benefit of preventing the devastating mental effects of iodine deficiency in children is well worth the increased incidence of thyroid disease in the adult population, but the large amounts of salt used in prepared foods and restaurant foods can cause an individual's intake of iodine to be much greater than intended. In addition, the use of iodine as a disinfectant contributes massively to iodine intake in North America. The amount of iodine found in milk and other dairy products, as the result of iodine use to disinfect cow's udders, is nothing short of astonishing, averaging 230 micrograms per quart of milk. This alone is sufficient to meet one's daily dietary needs, even in the absence of other iodine-containing foods, as the US RDA of iodine is only 150 micrograms per day. In spite of all of the above, however, most good multivitamin pills contain a full 150 micrograms of iodine per pill. A person who eats restaurant food or prepared food, drinks ordinary quantities of milk, and also takes a daily vitamin pill, will easily exceed the recommended intake of iodine several times over, day after day. Iodine is also used in large doses as a contrast agent for some medical imaging, but there is nothing that can be done about that.
Attached are some additional photos in case you would like to use them.

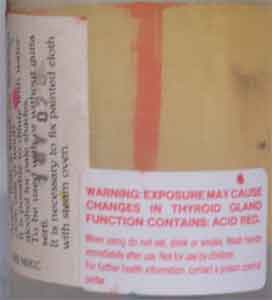
The
additional photos you sent gave me another clue as to the possible identity of
the thyroid-affecting dyes you had obtained from Sennelier. Here is another
iodine-containing dye: one of the photos you just sent was labeled with the
color 'Bengale', which reminded me of the Rose Bengal dye I used in my
dissertation work. Sure enough, Rose Bengal does contain
iodine:
Apparently
this dye has what is described as a 'weak goitrogenic effect'. It is also known
as "Acid Red 94". This is a strong candidate for being one of the dyes
included in the formulas of the old dyes whose bottles you found the warning
label on. The similarity in name is suggestive, though not proof of its
identity.
I recall that Rose Bengal was a lovely color. I was working
with bacteria, in my PhD work, so was unaware of thyroid issues at the time.
Rose Bengal has been used as a food coloring, somewhere, but it is not approved
for use as a food additive in the US. Unlike many of the dyes in my
research, Rose Bengal does not intercalate into DNA, and is therefore less
worrisome as a possible source of DNA damage.
(Please help support this web
site. Thank you.)
Tuesday, August 28, 2007
I'm trying to attain a bright, in-your-face red without luck, on 100% cotton. Would you happen to have a formula?
If you're using Procion MX type dyes, I like a mixture of Orange MX-2R (reactive orange 4) with Red MX-5B (reactive red 2), in approximately equal quantities. Do a small test to see if the relative amounts you mix of these two dyes look right to you. This mixture is particularly good for direct dye application, such as tie dye, because it does not produce the yellow halos you will get from mixing yellow MX-8G with red MX-8B.
If your colors are not coming out bright enough, you may want to use MORE dye. I like to use four teaspoons of dye per cup of water, when tie-dyeing. Old dye will also produce pinker reds, as will trying to dye fabric that is less than 100% cotton, or which has been treated for stain resistance.
A really good way to play with different color formulae is to look at Olli Niemitalo's wonderful Dye Mixer Applet. It won't tell you the precise amounts of each dye to use (this requires that you first determine for each dye how much to use to get a specific brightness indicated there), but it's a very quick, easy, and helpful way to get ideas.
Any magenta or fuchsia primary mixed with any bright yellow or orange can produce a pretty good red if mixed together in the right proportions. I do not like to use any dye mixtures as mixing primaries, but only the pure unmixed dye colors listed on "Which Procion MX colors are pure, and which mixtures?". Premixed dyes used as mixing primaries often result in duller colors.
You can try a series of several different proportions as a test to see how much of each to mix together. It's easiest to do this by mixing each dye in water and then combining different proportions of the resulting dye solutions. You can then easily mix different amounts of the two dyes, such as 1:1, 1:2, 2:1, 1:3, 3:1.... Unfortunately, you can't be certain of the final color of any dye mixture until the dye has fully set and the excess unattached dye has been washed out.
There is no widely available pure unmixed red Procion MX type dye, but every dye supplier sells pre-mixed reds, which can be useful timesavers, depending on your preferences. There is one pretty nearly true red pure unmixed Procion MX dye, Red MX-G or reactive red 5, but it is expensive, very hard to find (none of the usual dye suppliers carry it), and in no way superior to a mixture of Orange MX-2R with Red MX-5B. I bought some once from Standard Dye but did not end up using it much.
(Please help support this web site. Thank you.)
Monday, August 27, 2007
biography question
Some of your questions are answered on my web site, under "work". I am a molecular biologist, having received my PhD from Rice University in 1989. My dissertation (PDF) involved determining the mechanism by which, in the presence of oxygen and light, certain dyes and stains produce DNA damage and kill cells; this is called the photodynamic effect. The dyes I used in my graduate work are not related to the fiber reactive dyes I normally use, which are considerably safer to use.
I chose to study molecular biology, in what was then the Biology department at Rice (it has since been reorganized) because of my interests in molecular biology and biochemistry. My undergraduate degree was in biochemistry. I took a great many fine arts classes at the University of Maryland before settling on that course of study, intending at the time to get a degree in fine arts, instead, but my passion for scientific knowledge turned me away from that and toward the science degree.
When I took up dyeing as an art form, in my spare time while in graduate school, I was struck by how little information was available about the chemistry of the dyes in any form that was comprehensible to artists and craftsmen. At first I was frustrated by the different chemical properties of the different individual dyes, because I could see that they had a major effect on art made with them, and yet very little information was available concerning them. My scientific background enabled me to find and explain information that was otherwise useless to non-scientists. My web site developed as an ideal way to share the information I found. The more an artist knows about his or her materials, the more control there is over the work, and the better the results that are possible.
I'm afraid I have no history with the fashion world. Like most scientists, I have relatively little interest in fashion. What I care about is color and its use in art, especially art on textile fibers. I like to hand dye because I enjoy exploring color, and I do not like to be limited to the relatively small number of colors made available to us in clothing by the fashion industry each year. Most of what I dye are cotton and rayon garments for my family to wear, because I enjoy seeing my work in motion, rather than displayed static and lifeless on a wall or stored away.
(Please help support this web site. Thank you.)
Friday, August 10, 2007
is there is a store/site/provider of dyes etc that is the equivalent of the old store or better???
Message: in unpacking my textile stuff after decades in storage, i came across wonderful catalog from 1988 to store then in seattle--- cerulean blue ltd. i ran a net search to see if that store still existed. apparently not.....
all i came up with was industrial business of ceruleanblue and things that do not appear to be related to textile people.
is there is a store/site/provider of dyes etc that is the equivalent of the old store or better???
Cerulean Blue used to sell fabrics, fabric paints, and dyes, including Procion MX and Procion H dyes. Apparently, they went out of business some time ago. Since you are in California, you will probably want to buy your dyes from Dharma Trading Company, which is only a couple of hours away from you, in San Rafael. You will want to request a catalog. For eight-ounce jars of dye and larger, you can also order directly from Jacquard Products (Rupert, Gibbon, & Spider), in Healdsburg, California.
I maintain a list of many different suppliers for textile dyes and supplies listed on the page at the following link:
Thursday, August 09, 2007
I bought a red, dyed-in-India rayon skirt a couple of weeks ago, and whenever certain liquids drip onto or touch the fabric (such as cold condensation from a drinking glass), dark, ink-like stains appear.
What can I do? I love the skirt, but wearing it clearly comes with risk and frustration - I've gotten those 'ink stains' on it everytime I've worn it...
Have you ever heard of/encountered this phenomenon? Any info or guidance you can provide would be greatly appreciated.
I've never heard of that before. It's an interesting question. I wonder if the color change has anything to do with pH: laundry detergent generally has a high pH, and there is often some residue left in the fabric, so getting the fabric wet might be like increasing the pH. (If so, a drop of vinegar should be different from a drop of water, but I can't recommend that you try this, except perhaps on an inconpicuous inner seam; it's possible the results might not be good.)
Any dye that runs is a bad business. You don't want it on other clothes, and you don't want the loose dye particles getting on your skin. I would advise you to wash the skirt in HOT water to remove as much dye as possible; soaking in hot water will remove even more of the dye. The heat is the key. To set the dye, you could mail-order some Retayne (or check your local quilting supply store or fabric store first); this product will set most (but not all) commercial dyes. See Commercial Dye Fixatives for more information on Retayne.
(Please help support this web site. Thank you.)
Saturday, August 04, 2007
dyeing a child's yellow blanket to duplicate his blue one
I don’t know where to start?! The blanket is I think is 100% acrylic and knit. I read this doesn’t take color well. That’s OK if its not too bright because it is supposed to be a pale blue. Do I have to mix colors to get this? Like I said, I am such a novice. Any suggestions you have would be so greatly appreciated as I feel desperate to get his comfort back to him.
Usually it takes me a long time to work through the backlog of email I get, but I feel for your little boy with his lost blankie. Of course only part of the problem is the blankie's yellowness. The other part is that it is not worn as much as the other, so it feels different, and it does not smell right.
Since the blanket is yellow, you won't be able to get the same shade of blue. Adding blue dye to a yellow background creates green. Dye is transparent, there is no way around it. You cannot use bleach on acrylic, however. You might be able to remove some of the yellow with a product called Rit Color Remover. I recommend against using any brand of all-purpose dye, because they are not good for the uses most people put them to, but Rit Color Remover is an excellent product. Check your grocery store, pharmacy, and fabric store while looking for this product. It is displayed next to the Rit dye.
Not all dyes can be removed. Some yellows will be removed by Rit Color Remover, some will not. There's no way to tell until you try it. It will work better using the stovetop method than using the lower temperatures imposed by the washing machine method, but the washing machine method might work fine and is a lot less trouble. Be sure to buy enough boxes of Color Remover to fit your machine, if you try that first.
If you succeed in getting the yellow out, then you can dye the blanket blue. If the yellow will not come out, you will have to settle for green.
Acrylic cannot be dyed with ordinary dyes. All-purpose dye will not work, and neither will the good fiber reactive dyes we use on cotton. See "Dyeing Acrylic with Basic Dye". Acrylic can be dyed with basic dyes or with disperse dyes. I would recommend against basic dyes for a child's blankie. He might chew or suck on it, and these dyes tend to be more toxic. Instead, I would recommend that you use disperse dye, which you can buy by mail-order from PRO Chemical & Dye. Disperse dye on acrylic does not produce intense colors, but I imagine that you are looking for a pale to medium shade of blue. You can follow the "Immersion Dyeing Acrylic using PROsperse Disperse Dyes" instructions found at PRO Chem. You will need a large non-aluminum cooking pot to do your dyeing in. Follow the instructions closely. You can buy all of the dyes and chemicals you will need at http://www.prochemical.com.
There is no guarantee that you will be able to exactly duplicate the missing blankie, but there's a good chance you can make the yellow one more acceptable to him. Try the Color Remover first, because you can buy it in the grocery store or pharmacy. You cannot buy the dye you will need next locally, however, but must buy it by mail-order.
I hope that the missing blankie turns up.
(Please help support this web site. Thank you.)
Friday, August 03, 2007
When you say to soak the shirt in the soda ash before dying, do you mean before you tie it or just loosely and then tie it up wet?
Message: When you say to soak the shirt in the soda ash before dying, do you mean before you tie it or just loosely and then tie it up wet?
The shirt must contain soda ash when you add the dye. It does not matter to the dyeing process whether you choose to soak the shirt before or after tying.
Since soda ash is irritating to the hands, one should always wear gloves while handling it, but gloves make it more difficult to tie the strings or rubber bands. For this reason, I prefer to tie a clean dry shirt, or a shirt moistened only with plain water, so that I don't have to wear gloves. Only after I am finished tying do I dump the shirt in the bucket of soda ash and water.
(By the way, people have been writing to me recently asking about soda ash when they are dyeing with all-purpose dye, such as Rit or Tintex dye, which does not work with soda ash. Always use fiber reactive dye, such as Procion MX dye, for tie-dyeing cotton at room temperature.)
(Please help support this web site. Thank you.)
Can I use an ordinary steel pot to dye silk or wool in?
No, don't use a steel pot for dyeing with synthetic dyes.
The reason why you never want to dye in a steel pot is that iron acts as a mordant to "sadden" colors, that is, make them darker and duller. Many dyes (including synthetic dyes) which do not require mordants to stick to fabric can nonetheless form complexes with the iron atoms that will have undesirable effects on the dye's color.
You can use stainless steel because it contains other metals that bind tightly with the iron atoms to prevent them from reacting readily. Ordinary steel is too reactive to be suitable for use in dyeing, except when the dulling effects of the iron on the color are desired.
(Please help support this web site. Thank you.)
Thursday, August 02, 2007
i have searched for some one to dye my polyester top black but none seem to be in england who can i contact?
Message: i have searched for some one to dye my polyester top black but none seem to be in england who can i contact?
It's usually impossible to find anyone anywhere who is willing to redye a polyester garment. The problem is that results cannot be guaranteed, since the garment must be boiled in a special kind of dye for an hour, and most clothing will not tolerate such harsh treatment. See "Dyeing Polyester with Disperse Dyes".
It is much easier to find someone to custom dye a new silk or cotton garment.
(Please help support this web site. Thank you.)
Wednesday, August 01, 2007
I am very interested in tie and dye and have previously been using vat dyes and hydros. Would you know anyone who can supply these in small quantities?
Message: I am very interested in tie and dye and have previously been using vat dyes and hydros. Would you know anyone who can supply these in small quantities?
In the US, a good source for vat dyes is PRO Chemical & Dye. (They do ship internationally.) In Europe, you can order Indanthrene vat dyes from Granat Farvekompagniet in Denmark. (They speak English.)
Hydros is presumably sodium hydrosulfite, Na2O4S2, also known as sodium dithionite. Vat dye recipes generally call for lye (sodium hydroxide) and either sodium hydrosulfite, thiourea dioxide, or another sulfur-containing reducing agent. Lye, also known as caustic soda, is often used for clearing clogged drains. Sodium hydrosulfite is found in Rit Color Remover, is also available at photography supply shop for use in developing film, and is sold by Granat Farvekompagniet as sodium dithionite. Thiourea dioxide is sold by dye suppliers such as Fibercrafts in the UK, under the name of Spectralite.
Most tie-dyers prefer to use fiber reactive dyes, such as Procion MX dye, due to their much greater ease of use and the relative non-toxicity of the chemicals used with them (washing soda and urea). This type of dye is readily available by mail-order from most dye suppliers, including several in the UK. Check under "Europe" on my page of Sources for Dyeing Supplies Around the World. Good sources in the UK include Fibrecrafts, Kemtex, Omega Dyes, and Rainbow Silks.
(Please help support this web site. Thank you.)
