vacation notice
-Paula Burch
Friday, June 26, 2009
I am looking for a way to dye a white print a very pale pink
Message: I am looking for a way to dye a white print a very pale pink and keep the print intact. The fabric is 100% cotton. It is pittsburgh steeler fabric and I see on ebay a seller has a layette set in pink, but there is no pink available anywhere. Here is a link to the item and if you can give me any ideas, it would be greatly appreciated.
This looks like an extremely easy project to do. All dye is transparent, so the original colors will show right through the dye. The original colors will be affected depending on the intensity of the concentration of the dye you use.
If you overdye any print with a pale pink dye, the white parts will all turn pink, while the darker colors will remain darker. There will be no effect on the red or black portions of the print; the royal blue portions might pink up a slight lavender tinge, but probably not enough to be noticeable. Other colors, such as yellow or pale green, would be affected drastically by adding pink dye, but not the colors in the print you're considering.
You will find that any contrasting fiber content will tend to pick the dye up more or less strongly. For example, if you dye a cotton bag that has nylon straps, the straps may stay white, or they may pick up a more intense pink, depending on what dye and what recipe you are using. If there is elastic on the bottom of a fitted sheet, it will probably stay white when the fabric changes color.
Any dye that works on cotton will work for this project. However, I do not recommend the use of all-purpose dye if you can use fiber reactive dye, instead. Fiber reactive dye is much better. An all-purpose dye, such as Rit or Tintex, will tend to bleed when wet (though the problem will be minimal for a pale color like your choice of pink), and it will tend to fade considerably as the result of frequent washing. If you use a fiber reactive dye such as Procion MX dye, Tulip One Stop Fashion Dye, or Dylon Permanent dye, the dye will make a permanent chemical bond to the cotton fiber, so that it will not fade or bleed. You can buy these dyes from a crafts store or from a mail-order dye supplier such as Dharma Trading Company. You can also do very well by buying a good brand of tie-dye kit (not Rit) and using just the fuchsia dye from the kit on your fabric. You will want to use only a small amount of dye, in order to get a very pale pink. If you're not sure how much to use, use less, because it is much easier to dye a second time, to get a darker color, than it is to remove the color after you have dyed it too dark.
Before using the layette set for a baby, be sure to wash out all unattached excess dye first, as there is always excess dye loose on the fabric after dyeing. To do this with fiber reactive dye, wash once in cool water without detergent, and then wash two or three times with detergent in very hot water.
(Please help support this web site. Thank you.)
Thursday, June 25, 2009
I need information about reactive orange 84
Message: Good afternoon
My name is Hikari. I am a student who's doing some research about dye.
I've read your excellent site and I need your help.
I need information about reactive orange 84 and the important is the structure molecule of this dye.
I really honor if you want to help me.
Thank you so much.
Sincerely,
Hikari
Colour Index Reactive Orange 84 is described in the Chemical Book website as being an H-E type dye, CAS# 91261-29-9. Its molecular formula is C59H30I2N14Na8O26S8. The full chemical name gives all of the details of its molecular structure, if you can interpret it: 1,5-naphthalene disulfonic acid, 3,3-(2,2-disulfo1,1-biphenyl-4,4-diyl) bisimino(6-chloro-1,3,5-triazine-4,2-diyl) imino(1-hydroxy-3-sulfo- 6,2-naphthalene diyl)azo bis-, octasodium salt. I was not able to find an image showing this structure. As a student you may find it helpful to look at similar dye structures, in order to compare the complete chemical names to illustrations of the dye molecules they describe; my page about the structures of a related class of dyes, the dichlorotriazine, includes many similar structuress.
My web page on Procion H and H-E dyes, includes Reactive Orange 84 in its table and indicates that it is supplied for dye artists by PRO Chemical & Dye as their "Pro H-ER Real Orange". It is also supplied in larger quantities for industrial customers from various factories in Asia, not listed on my site. It is a bifunctional dye, classified as being a bis(aminochlorotriazine) dye. It is a hot water dye, requiring temperatures of 80°C (175°F) for immersion dyeing, or a half hour of steaming after it is applied by hand painting. There are links on the bottom of my Procion H and H-E page to instructions for using these dyes for small-scale hand dyeing. Industrial methods for using Reactive Orange 84 are nicely summarized in several graphs at the Camex company's page about their HE series dyes.
(Please help support this web site. Thank you.)
Wednesday, June 24, 2009
Deionized or Reverse Osmosis to Treat Hard Water?
Message: I have EXTREMELY hard water, so I will be bringing some in. I have access to R.O. water & R.O./DI water... which is better for mixing Dyes? (RO= Revese Osmosis, & RO/DI is Revers Osmosis/Deionized)
Do you know what contaminants are in your water supply to worry about? The hardness minerals in water are calcium and magnesium. Metals such as iron and copper will also interfere with dyeing; iron in particular will "sadden" the colors of many dyes, making them duller, darker, and less brilliant.
You do not have to bring in water that is treated by reverse osmosis or deionization if you can treat your water with the water softener chemical, sodium hexametaphosphate. (See "Dyeing with hard water: water softeners, distilled water, and spring water".) Your dye supplier should carry this substance. Dharma Trading Company calls it "Water Softener", PRO Chemical & Dye calls it "Metaphos", and Jacquard Products and Fibrecrafts call it "Calgon". This chemical is sold in powder form: do not substitute liquid Calgon brand products! They contain an entirely different type of chemical, the polycarboxylates, which may interfere with dyeing. The amount of sodium hexametaphosphate that you should use depends on how much hardness is in your water. See "how much metaphos to use" in the Dye Forum.
Whether a system that is labeled "reverse osmosis" is superior or inferior to one labeled "reverse osmosis/deionizing" depends on how much of the hardness minerals or other problem minerals are present. Check the information provided by the manufacturers. You want a system that will remove the vast majority of any calcium and/or magnesium ions. A home deionizer will remove polyvalent ions such as calcium and magnesium (both of which have a charge per ion of +2) by substituting sodium ions. If you have a serious problem with iron, contact the manufacturer and ask how much of the iron in the water their systems will remove.
Distilled water is the best, as far as freedom from unwanted minerals is concerned, but it is almost never necessary to use distilled water.
(Please help support this web site. Thank you.)
Tuesday, June 23, 2009
looking for sources of silk cording, silk ribbon and silk bias ribbon
Hi Ann,
Test Fabrics has some silk ribbon. I haven't seen it:
http://www.testfabrics.com/products/tapesthreads.htm
Aurora Silk has something they call Lustre Cord:
http://www.aurorasilk.com/yarns_and_threads/
threads/spun_silk_thread/lustre_2ply.html
Treenway Silks has silk ribbons:
http://www.treenwaysilks.com/ribbons.html
Cam Creations sells silk cording and bias-cut silk ribbon:
http://www.silkribbon.com/silk_cord.htm
Their selection is much wider than any of the others.
I have not purchased any of these silk ribbons or cording, so I'd be interested to know any impressions you have, if you do order any of them.
(Please help support this web site. Thank you.)
Monday, June 22, 2009
How long does Anti-Chlor last?
Message: Hi there! I'm planning on making bleach designs on a lot of shirts and I've done a lot of web research on the topic. I'm 99% done. All I'm wondering, is for the neutralizing step with the anti-chlor, if I have a bucket with the water/anti-chlor mix in it, about how many shirts do you think I could put in it at a time, or individually, and how many times could I use the same bucket of solution? Does it need to be refreshed with clean water and new anti-chlor from time to time? You seem to have all the answers so I hope you can help me. I can't find any suggestions anywhere on the web.
Hi Chrissy,
The Anti-Chlor does get used up as you go. You should, however, rinse your shirts carefully in plain water before placing them into the Anti-Chlor bath. This will considerably reduce the amount of hypochlorite that goes into your neutralizing bath. Otherwise, you would need to use far more Anti-Chlor. Using a larger quantity of Anti-Chlor would also mean that you would experience far more of the reaction products in your air, which could be a problem if you have asthma. Many people with asthma are sensitive to sulfites.
ProChem says to start with one teaspoon (2.2 grams) of their Anti-Chlor (sodium metabisulfite) per 2.5 gallons of water. (If you use Bleach Stop, or thiosulfate, instead of Anti-chlor, you'll need to use a much greater quantity, 30 grams per gallon.) This is described on my page on "How can I neutralize the damaging effects of chlorine bleach?".
The question of how frequently you would need to refresh your Anti-Chlor bath depends on how much stuff you are neutralizing. To get a lower limit of how far the Anti-Chlor will go, let's see how much Anti-Chlor it would take to react with a certain amount of unrinsed household bleach. Unfortunately, I have been unable to determine the exact chemical reaction between the bisulfite of the Anti-Chlor and the hypochlorite of the bleach, but let's assume that one molecule of Anti-Chlor will react with one molecule of hypochlorite. In one-quarter cup of household bleach, at 5%, there will be about 3 grams of sodium hypochlorite molecules. The number of hypochlorite molecules in one-quarter cup (59 ml) of household bleach is about 0.04 moles (a unit of a certain number of molecules), which works out to be the same as the number of moles of sodium metabisulfite in 7.5 grams of Anti-Chlor. How much household bleach did you start out with? How much household bleach was used, on average, in producing each shirt?
Practically, though, if you rinse your shirts well, before putting them into the Anti-Chlor, you will need only a tiny fraction as much Anti-Chlor to neutralize it.
You cannot save your Anti-Chlor solution from one day to another, so these calculations are only for when you are working with a large number of items at once. The sodium metabisulfite in the Anti-Chlor not only reacts with the hypochlorite in the bleach, it also reacts with the oxygen in the air. This means that Anti-Chlor solutions "go bad" after a while and should be prepared just before use. This fact is convenient when you are preparing water for use in an aquarium, because water that has been treated with Anti-Chlor to remove the chlorine so that fish can live in it must also be free of Anti-Chlor itself; simple exposure to air will break down the sodium metabisulfite.
If you have reason to avoid the use of sulfites, then a perfectly effective and safe alternative is to use the 3% hydrogen peroxide which is sold at pharmacies for use as an antiseptic. The reaction between hydrogen peroxide and hypochlorite bleach produces safe chloride ions in the water, plus it produces water molecules, and oxygen molecules (the source of the bubbles you see). Hydrogen peroxide is a little less economical than Anti-Chlor, but not hugely expensive, and in my experience protects the fabric extremely well against continued degradation by the hypochlorite.
I recommend against the outdated practice of using an acid such as vinegar to neutralize bleach, because it may produce chemicals that are even more caustic to the fabric, or even to your lungs; stronger acids than vinegar are known to produce dangerous amounts of chlorine gas when they react with hypochlorite.
(Please help support this web site. Thank you.)
Sunday, June 21, 2009
I am doing tie dye shirts with 150 preschool and elementary age kids
Message: I am doing tie dye shirts with 150 preschool and elementary age kids. I was wondering if you could guestimate the amount of time I should allow for the craft? Also, do you have any idea how many ozs or lbs of dye I would need to purchase to dye 150 youth size shirts? And lastly, if I break this up into small sessions will it hurt the outcome, such as 1st day, pass out shirts and have them chose design and rubberband their shirt and 2nd day soak in soda ash and dye shirts? Thank you so much for your very informative site. I have never tie dyed before myself but after reading your information I am really excited about this project.
Before you do this project, it would be a very good idea for you to try a little tie-dyeing at home, just to make sure you have a handle on how to do everything. It's not difficult, but practice can be helpful. Try dyeing a shirt for yourself.
I think that breaking this project up into multiple days is an excellent idea.
Day one, have them label their shirts some way, or you do it for them. You can buy brass (non-rusting!) safety pins and use them to attach tags, which you can cut out of Tyvek envelopes and label with permanent black Sharpie markers, or you can mark on the tag inside the collar of the shirt. (Look for shirts that have tags sewn in, as opposed to the ones with tags printed on the inside of the neck; buy only 100% cotton shirts, which are not stain-resistant.)
They can fold and tie or rubber-band their shirts either dry, or moistened with plain water. Moistening them with water helps in tying more detail designs, but it's not required. I think you should just have them tie the shirts while they are dry, to simplify things. Simple ties are fine: bullseye, stripes, or random crumple.
In the evening, a day or two before applying the dye, you should mix up the dye powders yourself. Wear a dust mask or respirator so that you do not breathe the dye powder. It's not horribly toxic, but you can develop an allergy to the dye if you are careless. The dye mixtures will stay good for a week at room temperature, as long as they are not exposed to any soda ash at all. You will keep the soda ash separate. (If you buy a Tulip brand tie-dye kit, the soda ash is mixed in with the dye, in which case the dye must be used immediately.)
Be sure to advise the parents to send their children in clothes that will not be a great loss if they get stained with dye. You can buy disposable plastic aprons to help protect the children's clothes. Don't forget to buy at least one pair of size extra-small plastic or latex gloves for each child! You will need plastic to protect the tables, or better yet go outside and do the dyeing on the lawn if there is one.
On the second day or the project (which does not have to be the very next day after tying the dry shirts), soak the shirts in the soda ash, squeeze out the extra water, and apply the dye. Have a large number of plastic bags available. Ziplock bags are excellent; plastic grocery bags usually have holes in the bottom. After the dye is applied, pop each shirt into a plastic bag and keep it in warm place (or outside in the summer heat) overnight, at 70°F or warmer. It's okay to leave them an extra day if necessary for your schedule.
After the dye has had plenty of time to react with the shirts and the soda ash, take them to a washing machine. Washing 150 shirts out by hand would be terrible. You may dump a washing-machine-load into a washer full of cool water, all at once. I like to use a pair of children's blunt-ended scissors to cut the rubber bands off as I dump them in. I do not like to hand rinse each of a large number of shirts. After one cool rinse, wash the shirts at least twice in hot water with detergent or Synthrapol. Dry them in a clothes dryer if one is available.
On the third day, you can hand out the shirts to their artists. This is when you realize how important the labeling step was.
You can estimate that 150 youth-size t-shirts will take approximately as much dye as 75 adult-sized t-shirts. A "big group" tie-dye kit from Dharma will be more than enough, as it can produce up to 100 adult sized t-shirts; it costs about $70, and contains twelve ounces of Procion MX type dye powder. Alternatively, you could buy a number of Jacquard or Tulip tie-dyeing kits from a local crafts store, but the smaller kits will add up to a greater expense. (Avoid the Rit tie-dye kits!) The shirts sold by crafts stores are usually overpriced and often 50% polyester, which you do not want; look for 100% cotton. You can buy youth-size t-shirts for about $2 each from Dharma.
(Please help support this web site. Thank you.)
Saturday, June 20, 2009
Washing instructions: "dry clean only." Can I dye it?
Message: I want to dye a dress that is 55% linen and 45% rayon. Washing instructions: "dry clean only." It is currently a light, light pastel purple and I'd like to dye it a bright hot pink. Is this something I could do? If so, could you recommend a cold water dye that might work? Do you think I can use my washing machine on delicate cycle? Or do you think I'd be more successful hand dyeing?
Whenever you dye something, you have to wash it a lot. You must prewash it to remove any invisible stains that will repel dye, and you have to wash it several times afterwards to remove all of the loose extra dye, as otherwise the excess dye will rub off on you, the furniture, your purse, and anything else the garment touches.
This means that you absolutely cannot dye anything that is not washable.
If you are willing to risk destroying the garment, go ahead and wash it. You can use the delicate cycle. Sometimes items marked "dry clean only" survive washing very well. I have successfully machine-washed both linen and rayon dresses. If your dress still looks fine after you've washed it, you can then try dyeing it.
If your dress is unlined, it is likely to do well when washed. There's a small though real chance that part of the dress will shred, in which case you'll have to throw it away. If the dress is lined, however, there's a near-total chance that the linen/rayon outside of the dress will shrink a bit, while the synthetic lining (whose fiber content is almost never noted on the label) will stay the original size. This will ruin the shape and fit of the dress. It's not worth the risk to try washing a dry-clean-only dress if it is lined.
Note that the seams that hold the dress together are made of polyester thread, which means that they will remain their original color even after dyeing. If the stitching is very visible, the results might look peculiar. Look at your dress critically and decide whether or not it will be a problem for it to have pastel purple stitching on a hot pink dress.
If your dress survives washing, then you can dye it in the washing machine on the delicate cycle, resettng as needed to allow plenty of time for the dye to react with the fabric. I recommend that you use a cool water fiber reactive dye, such as Procion MX dye. See the page, "How can I dye clothing or fabric in the washing machine?". If you are in Europe or Australia, consider using Dylon Machine Dye, which is designed for use in front-loading washing machines; this dye is not available in North America.
(Please help support this web site. Thank you.)
Friday, June 19, 2009
whites are turning blue or purple in tie-dyeing
Message: I have searched your website and I have tried everything. I need help. We are in our 3rd year of dyeing so we are still really new at this. We are getting very busy so we are making a lot of shirts. Our shirts are coming out muddy. By muddy I mean the white is tinted blue or purple but not by much. We were following the instructions on the True Tie Dye videos. After visiting your website a few weeks ago, we quit using the synthrapol and we turned up our hot water heater. We do a cold rinse first then spin it out then fill it with hot water and let it do a double rinse and spin. The shirts look better but the white still isn't as white as it should be. So the today I started making the doubel rinses be warm instead of cold. We are using the Procion MX dyes from Dharma and our water is reading over 140 degrees right out of the tap. Is there anything else we can try to get the white whiter!!! Thanks for your website. You are a lifesaver!
Most importantly, are you allowing your dyes to react with the fabric for long enough? To avoid permanent backstaining, it is best to allow extra time, at warm enough temperatures, that all of the dye molecules have fully reacted. Some will react with the fabric, and some with the water, but if you allow enough time, all will have reacted. This is very important because it prevents still-reactive dye from permanently staining another part of the shirt when you wash it out. If you allow overnight at 70°F or higher, and keep the fabric moist with either plastic wrap or by including urea in your dye mixtures, then all of the dye should have reacted. If, however, you wash the shirts out as soon as they've gotten an intense enough color, then some of the dye will still be active, and can easily stain another part of the shirts. If the temperature falls below 70°F (21°C) during the reaction time, you should find a way to increase the temperature.
When dye that has fully reacted with the water stains another part of the shirts, it's via very weak bonds which can be washed out in hot water. When dye is allowed to react with the fabric in the wrong place, though, the color is permanent and cannot be removed even by boiling.
My next suggestion would be to turn off the washer before it starts the cool or warm rinse, and repeat the hot washing. It's not that the cold or warm rinse does any harm, but it's a waste of water until you've completed the hot water washing. You might need to do more washing. Try soaking in hot water and then washing again in hot water.
A third important consideration: do you use water softener? Hard water makes it much harder to rinse out the dyes. Use the sodium hexametaphosphate that Dharma sells or the Calgon that Jacquard Products sells, or the Metaphos that ProChem sells. Do not use liquid Calgon from the grocery store, as it contains different chemicals that can interfere with dyeing.
Another issue to consider is starch in the fabric. Are you using PFD shirts from Dharma? They are usually very reliable. Shirts from other sources might contain starch or another problematic sizing, if they're not labeled PFD.
Are you prewashing your shirts in hot water before you dye them? That probably won't make any difference to how white the whites stay, though. And you are using soda ash, of course, right? Synthrapol is fine to use.
I hope one or more of these ideas help. Please let me know.
(Please help support this web site. Thank you.)
Thursday, June 18, 2009
Can I dye a waterproof tent?
—ADVERTISEMENTS—
Message: Hi I have a tent made of 190T polyester fabric with waterproof PU coating and am wondering if I would be able to dye it as I am sick of the colour. Thanks.
No, you can't. Unfortunately, it is completely impossible to dye anything that is waterproof. You can't even paint it with fabric paints, because the water-resistant coating will resist all dyes and paints.
You will have to put up with that color until you can buy a new tent. It might be worth looking for a previously-used tent that you like better.
(Please help support this web site. Thank you.)
Wednesday, June 17, 2009
Trying to find a store or company or a dry cleaners that dyes clothing
Message: I am trying to find a store or company, or a dry cleaners, in metro Detroit that dyes clothing. It's hard to find, can you help me?
I do not know of any company in Detroit area that does custom dyeing. I know of several in other cities. See my page answering "Where can I find someone to dye my clothing for me?".
Contact a custom dyer on that list, explaining what the fiber content of your clothing is, and what the original color and the desired color are. Not all garments can be redyed; most dyers refuse to dye synthetic fibers, such as polyester. You can find out how much it will cost, by phone or email, and mail the garment to them. They will dye it and mail it back to you.
(Please help support this web site. Thank you.)
Tuesday, June 16, 2009
Are there any professional or famous tie-dyeing artists?
—ADVERTISEMENTS—
Tom Rolofson and Martine Purdy's
Advanced Tie Dye Techniques: Making Shapes and Mandalas
Message: Hello there. I'm doing the first year of GCSE Craft and Design and part of the course is to design and make a bag with a selected theme. I have chosen my the to be tie-dye. However, I desperately need to know if there are any professional or famous tie-dyeing artists/designers.
There are a great many professional tie-dyers working on a small scale, often selling their work directly to their customers. For examples, look at my list on the page, "Where can I find someone to dye my clothing for me?", and the tie-dye section of my "Links to Other Hand Dyers' web sites" page.
You will want to examine the discussions on the history of tie-dyeing in the Dye Forum; see Tie Dye History and Tie-dye history in the West. There are quotes from media sources contemporary to the tie-dyers of the 1960s. A Time magazine article from 1970 mentions John Sebastian, Tie-Dye Annie, Maureen Mubeem, Bert Bliss, and Will and Eileen Richardson. There are also quotes from a 1970 interview with Maureen Mubeem.
A more recently famous tie-dyer would be Michael Fowler. He authored the DVD entitled "The Art of Tie Dye"; the blurb for this video mentions that "he has been commissioned to make tie-dye pieces for Shure Microphones, Volkswagen of America, Austin Powers, Sony Entertainment, Rolling Stone and Musician Magazines, as well as the BP-Amoco Corporation". He created a tie-dye community around the website he founded, Tie-dyed.com, then abruptly disappeared from public view around the end of 2005. The DVD is still in demand, although it is out of print; used copies are difficult to come by.
Other tie-dyers known for their publications include Tom Rolofson and Martine Purdy, authors of the "Tie Dye 101" and "Advanced Tie Dye Techniques" DVDs, and Brad Garrett, author of the Phat Dyes series of tie-dyeing videos. These videos go into far more detail than any of the books available on the subject. An earlier tie-dyer known for her publishing on the subject is Virginia Gleser, author of a simple introductory book, "Tie Dye: Back by Popular Demand"; she was a member of The Farm, a commune in Tennessee where some of today's most popular tie-dye patterns were created, where she and her husband, Robert Gleser, started tie-dyeing in 1979.
(Please help support this web site. Thank you.)
Monday, June 15, 2009
I want to dye a white king size duvet cover a dark red colour
Message: I want to dye a white king size duvet cover a dark red colour. How much dye would I need to use - and is it better to do it by hand?
A piece that large is going to be a difficult job to do, if you want a single smooth solid color. It's difficult to apply dye evenly to something too big to be easily stirred, and then you will have to wash out the excess dye afterwards. It's not something I can recommend that you do in a bathtub.
How much dye will you need? That depends on what kind of dye you use, and what fiber your duvet cover is made of. It doesn't matter how much dye you use, if it's the wrong type of dye for your fiber type. Weigh your cover, and then you can adapt a dye recipe to suit the weight of your fabric.
It is most important to figure out what fiber the duvet cover's fabric is made of, and choose your dye type based on that. It's pretty easy to dye cotton or silk, and if you use the right kind of dye plus some acid, you can dye wool or nylon, but there are some fibers that are not practical to dye. I do not recommend that you bother trying to dye a duvet cover made of a synthetic fiber such as polyester or acetate. I also do not recommend dyeing anything that is treated to be stain-resistant or permanent-press.
Can you fit the duvet cover in your washing machine? If so, that will be by far the best way to dye it, if you have a top-loader washing machine. See "How can I dye clothing or fabric in the washing machine?".
If your duvet cover is made of cotton, linen, rayon, or dyeable bamboo, you can use Procion MX dyes. Other fibers require different dyes, most of which can be applied only by boiling them with the fabric, which makes them impractical for a large piece like yours. See "Choosing the Right Dye for your Fiber".
(Please help support this web site. Thank you.)
Sunday, June 14, 2009
I have a dark green silk taffeta dress that I want to dye black
thanks for you time
If it's washable, you can dye it with either acid dyes (such as are used for wool and nylon) or with reactive dyes (such as are used for tie-dyeing cotton). If it's not washable, you will not be able to dye it.
Most black reactive dyes will not dye a true black on silk, since they are calibrated for use on cotton, but you can mail order a Procion MX dye called "Silk Black" from PRO Chemical & Dye, and use it with soda ash in the washing machine. See "How can I dye clothing or fabric in the washing machine?".
There are several different types of acid dye that you can use. Dyeing in the washing machine would be easiest and would help to prevent uneven splotchy dyeing. You can find instructions for washing-machine dyeing with Jacquard Acid Dye on the Jacquard Products web site. You will need three ounces of the Jacquard Black Acid Dye powder to dye a pound of silk. Lighter colros do not require nearly as much dye.
All-purpose dye will also work on silk. A problem of which people frequently complain, however, for all-purpose dye, is that the black dye produces a color other than black, such as dark purple. All-purpose dye has the advantage of being easy to find. Common brands available in local stores include Rit All Purpose Tint and Dye and Tintex Easy Fabric Dye. These dyes are known for bleeding badly whenever the item is washed, so if you use one of them, be sure in the future to always hand wash the dress separately, in cool water, or have it dry cleaned.
(Please help support this web site. Thank you.)
Saturday, June 13, 2009
IS there a way to dye red cloth rose petals white?
—ADVERTISEMENTS—
Message: Ok I have a question. My sister bought some flower petals that is made of cloth for her daughter's wedding and they are red. Is there a way she can dye them white?? Thanks Annette
No. Buy new ones that are the color you want, or skip them altogether.
There is no dye that will turn red into white. Dye is transparent, so the original color will always show through any additional layers of dye.
In some cases red dye can be removed from fabric, but it's impossible to predict how well it will work. Many dyes cannot be removed at all, or only partially, and attempting to remove some dyes will produce a weird color, such as brown or dull yellow.
The artificial flower petals are probably made of polyester, even if they are described as silk. They may be colored with dyes, which are sometimes but not always dischargable, or they may be colored with pigments, which usually are not. The synthetic-fiber fabric will be damaged if you try to bleach them. It might be possible to remove the coloring by cooking them on the stovetop with a product called Rit Color Remover, but it would be a lot of trouble and is not guaranteed to work. Even if it removes the red, it may lave a color other than white.
I think that real flower petals, obtained from a florist, would be more appealing than synthetic fabric flower petals, but they'd be more trouble, too, since you cannot obtain them much in advance.
(Please help support this web site. Thank you.)
Friday, June 12, 2009
iron oxides and indigo for painting fabric
—ADVERTISEMENTS—
Instant Indigo
Instant indigo is natural indigo processed by a new method from India. The indigo has been pre-reduced and then freeze--dried into a crystal. As long as you keep it dry, it will keep indefinitely. It is easy to use and gives deep, wonderful colors. It is suitable for all natural fibers and will also dye many synthetics such as nylon, tencel and rayon. In addition, it is very cost effective. Make sure to keep this dye in your freezer if you live in a humid climate.
Message: Hi, I am trying to locate earth oxides/indigo for painting... I previously purchased from Dyeworks and they no longer carry them...any suggestions as to who/where I can get them?
I'm sorry to hear that DyeWorks/Table Rock Llamas is no longer selling pigments for painting on soy-treated fabric. Fortunately, there are other sources from which you can buy these or similar pigments.
You can buy freeze-dried instant (pre-reduced) indigo from Paradise Dyeworks. You can also buy it from Aurora Silk, which has instructions for painting with instant indigo on their site. Indigo is less wash-resistant when used as a paint than when applied in a reducing vat.
Dick Blick Art Materials sells sets of natural earth pigments, intended for mixing your own oil paint; I imagine that some of these are the same as what you used before. They also sell chemically-synthesized pigments, some of which are of historical interest, so you will need to look closely to see which are the ones you want to use. Their Eight-color Historic pigment set
- Yellow Ochre (Warm Gold Shade)
- Raw Sienna (Warm Shade)
- Burnt Sienna (Deep Warm Shade)
- Genuine Indian Red (Hematite)
- Burnt Umber (Reddish Brown) from Cyprus
- Caput Mortuum (Reddish Violet)
- Burnt Umber (Very Dark)
- Raw Umber (Very Dark Neutral Shade)
You can find more information about the contents of these and other pigments in the MSDS pages at Blick. The pigments sold by Dick Blick are actually made by Sinopia Pigments; be sure to look at their website.
As you know, pigments cannot be used for painting fabric, if the fabric is to be washed, unless the pigments are fixed in place, as in fabric paints, by using some sort of polymer binder to "glue" them to the fabric. One can either use acrylic clear fabric paint extender, such as the Neopaque brand of
Colorless Flowable Fabric Paint Extender, or use the traditional Japanese method of treating the fabric with fresh home-made soy milk, which, after aging, functions in very much the same way, though its washfastness is less reliable. Store-bought soy milk is not recommended for this purpose, because it does not work nearly as well as freshly home-made soy milk.
Here are the old instructions from DyeWorks for using freshly made soy milk to fix their earth oxides on the fabric:
To Use Earth Oxides:
"Step 1: Make soy milk. Follow instructions from the “Soy Milk Kit”. Make enough to treat the fabric, and reserve some to mix with the Earth Oxides the following day. Generally, process ¼ cup soy beans into soy milk for every two yards of fabric you intend to design.
"Step 2: Wash and dry your fabric. Brush on soy milk and hang to dry 24 hours. (Store reserved soy milk in refrigerator, covered.)( Use a table covered in clear, 6 mil plastic.) (Alternative: Use a traditional Japanese Harita/Shinshi set-up outdoors, suspended between two trees, poles, etc.)
"Step 3: Place 2 heaping teaspoons Earth Oxide into a plastic container. Add in ½ cup soy milk, stir well and let stand 15 minutes. Stir again, and add additional soy milk to reach a ‘rich chocolate milk’ consistence. Dip in your wetted paint brush and begin painting with your Earth Oxide upon your dry fabric. (Store your remaining Earth Oxides, covered in the refrigerator for up to 24 hours.)
"Alternate Step 3: Place 2 heaping teaspoons Earth Oxide into a plastic container. Add in ½ cup soy milk, stir well and let stand 15 minutes. Stir again. Add in about ¼ cup prepared gum tragacanth solution. (See ‘Gum Tragacanth’ instructions.) Evaluate the consistence and adjust with additional gum tragacanth solution, to your taste. This solution is ready for silk screen, thermal fax or stencil printing, as well as brushing on for stamps and special effects with found objects, fish, handprints, etc. (Store your remaining Earth Oxides, covered in the refrigerator for up to 24 hours.)
"Step 4: Allow your fabric to dry in place for 24 hours. (You may hang it up to dry once the danger of possible running has passed.)
"Step 5: Fold and steam (stuffed in a giant Ziploc with the air pushed out. We find this technique sufficient.) for two hours. Let stand to cool, then hang for an hour. Wash in the washing machine, once or twice is usually sufficient. Check your wash water, and continue washing cycles until it is ‘clean’. We recommend Synthropol in each washing round.
"Alternate Step 5: Hang and air cure your fabric for 2 weeks. Wash as directed above.
"Next Design Steps: You are ready for your next steps now. Your fabric will be colorfast.
You may mordant and dye the cloth now.
You may dip in indigo.
You may print with textile paints.
You may print with a solution of iron and gum tragacanth (See Iron for instructions.) to give another layer of shade to your Earth Oxides.
"Clean-Up Hints: Be sure to wash off the soy milk and Earth Oxide remnants which may remain on your plastic table cover. Be sure to wash your brushes of soy milk with Dr. Bronner’s soap, for the most gentle and thorough removal of the soy oils."
(Please help support this web site. Thank you.)
Thursday, June 11, 2009
Wax and dye questions for teaching batik in summer camp
Message: I teach camp crafts. I would like to do a cross with batik tie dying this year. Would you recommend the jacquard tie dye kit mentioned on your site for this (Or the steve spangler science kit)? They have lists of what comes, but doesn't mention whether you are mixing dye with urea and what the dye fixer is.
I also wondered what the ratio of beeswax to paraffin you recommend for batik (I pour candles so I have this stuff). Would you just use paint brushes and skit the tjanting pens with High school kids?
Thank you for your time. I hope I didn't ask a question you already answered on your website.
The Jacquard tie dyeing kit is an excellent choice for batik. I'm not sure whether it contains urea, which is optional, but it does contain soda ash as a fixative, packaged separately from the dye. (Some kits include soda ash already mixed with the dye, which works fine only if you use the dye immediately after mixing it with the water.) The Steve Spangler tie-dyeing kit also looks excellent; their "Tie Dye Fixer" must be soda ash. For the best prices and a wider range of color choices, consider mail-ordering a tie-dye kit from PRO Chemical & Dye or from Dharma Trading Company.
A typical ratio of beeswax to paraffin is 15% beeswax to 85% paraffin. Some premixed batik waxes contain 50% of each. Use the larger amount of paraffin for more "crackle"; use more beeswax for smoother lines. You can substitute microcrystalline wax for beeswax to save money, but then there's a little more difficulty in removing the wax since the melting point is higher.
You might want to try something relatively new, which is soy wax, as a substitute for beeswax and paraffin. It must be melted and applied in just the same way as batik wax, but the wax does not have to be boiled out when you are finished. It can be removed by washing it in a bucket with detergent in hot water. Some dyers throw the waxy shirts directly into a hot washing machine, but if you use an inadequate amount of detergent, so that the soy wax is not dissolved, the wax can clog your pipes, so I prefer a prewashing with detergent in a bucket first, to be able to see that the detergent has solubilized the wax. The soy wax to use is the hardest type, made for pillar-type candles, not for container candles; you can buy the right kind of wax from a good dye supplier.
Paint brushes work fine. Tjantings allow for some more interesting details, such as written words, but they do drip a lot. It's a good idea to place something, such as sheets of cardstock, over sections of the fabric that are not being waxed at the moment, to avoid unwanted drips.
Every one of your students must be well-behaved, and not inclined to horse around physically, since hot wax can be very dangerous, even more dangerous than boiling water. If you have a class with any impulsive students, I recommend using Elmer's Washable Blue School Glue Gel, instead of wax, to make your resist designs. Use a hair dryer, if necessary, to get the glue gel absolutely dry before applying dye, and apply dye directly by dripping or squirting it on, rather than in a bucket, so that the glue gel does not transfer from one section of the fabric to another after the dye moistens it. Instead of presoaking the fabric in soda ash, as you do in tie-dyeing, add the soda ash directly to the dye immediately before squirting it on, because soaking the glue gel designs in the soda ash will cause them to float away before you apply the dye. You do not get the cracks in the glue gel designs that you get with wax, but the applicator tip makes it very easy to add complex details to the designs, and it's wonderful to completely avoid the dangers of hot wax, burns, fire danger, and wax fumes.
(Please help support this web site. Thank you.)
Wednesday, June 10, 2009
Dyeing polyester/polyamide blend in the Netherlands
Message: Ik wil een jas verven van Polyester en polyamide. Welke verfmiddelen heb ik hiervoor nodig en waar zijn te koop in omgeving Breda. Graag in het Nederlands antwoord en via mijn e-mail a.u.b. Groetjes
Fortunately, I don't need to be able to speak Dutch to answer this question. To dye polyester, you will need to boil the garment with disperse dye. You can buy this dye in your area from Zijdelings; see http://www.zijdelings.eu . See their "DISPERSE VERF voor synthetische stoffen".
To dye polyamide (nylon), you can use disperse dye or you can use acid dye. Both types of dye will work on nylon. We usually prefer acid dye for 100% polyamide. However, for a garment that contains both polyester and polyamide, disperse dye is preferable since it will color both materials.
In addition to the special dyes, you will need a very large stainless steel cooking pot, large enough for the garment to move freely. It takes a considerable amount of boiling to dye polyester. You cannot dye polyester at temperatures below 100°C.
For more information, see my page entitled "Dyeing Polyester with Disperse Dyes".
Tuesday, June 09, 2009
What do you think it would take to dye a nylon parachute?
—ADVERTISEMENT—
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!
Message: What do you think it would take to dye a nylon parachute?
It would take a huge quantity of acid dye, plus a truly vast cooking pot (or dyeing machine) so that you can heat the nylon in the dye.
Uncoated nylon, if it is free of oils and other finishes or contaminants, is easy to dye with almost any acid dye, in the presence of a mild acid such as vinegar, but heat is required for the best results. It's hard to imagine a container large enough to immerse a parachute; how big do you think it would have to be, in volume?
Among the best dyes for nylon are the Nylomine dyes, many of which are also sold in PRO Chemical & Dye's line of WashFast Acid Dyes. Most other acid dyes, which are dyes intended for use on wool, will also work on nylon, because nylon, like wool, is a polyamide fiber. See my page, "How to dye nylon or polyamide".
Some dyeing of the nylon can take place in merely hot water, such as in a washing machine, but you'll still have to immerse it. Can you find a container large enough to immerse it, and a source for that large a quantity of hot water? Will it fit in an ordinary washing machine, or will you have to find a much larger commercial machine?
If you airbrush or spray a huge piece of clean nylon with acid dye, you can wrap it up and then steam it, in a large pot or some industrial equivalent, to set the dye. Moisture is required during the heat-setting process, for any true dye. If you use dry heat, you must keep the nylon wet with the dye; dry dye will work only if moisture from the steam can contact every bit of the nylon.
To commercially dye a large piece of nylon, you can contact one of the custom dyeing companies listed on my page entitled "Where can I find someone to dye my clothing for me?". Not all dyers are interested in dyeing synthetic materials such as nylon, but many more will dye nylon than will try dyeing polyester.
Alternatively, and again only if the nylon is free of any sort of oil or other surface finish (waterproofing makes it impossible to dye), you can use fabric paint to "pigment dye" nylon. Don't use ordinary artists' acrylic paints, or house paints, because these will produce a stiff and scratchy surface, and will not stick to the fabric as well. You can add a product called "fabric medium" to acrylic paint to turn it into fabric paint, or you can buy fabric paint in jars. I recommend you buy from suppliers that can sell you gallon-sized jars of fabric paint, not just the little 8-ounce jars (which many crafts stores carry). Dharma Trading Company sells several different fabric paints that are recommended by their manufacturers for synthetic as well as natural fibers.
In addition to being concerned about whether there are any oils or other contaminants on the nylon that will block the action of your dye or paint, you also should be concerned as to whether your material is truly nylon. My mother bought yarn that was sold as "parachute silk" which turned out to be not silk, and not nylon, but rayon, which is a cellulose fiber, and which dyes completely differently than nylon. Polyester is another possibility, much more difficult to dye than nylon. To determine the fiber content, you may snip off a tiny bit of material and carefully subject it to a burn test. Two excellent resources for interpreting the results of a fiber burn test are Griffin Dyeworks' Burn Test page and Ditzy Prints' Fiber Burn Chart.
To determine whether there are finishes on the nylon that will cause problems, you will probably have to do a test dyeing. Unfortunately, these are very common, so common that it's best to look for sources of PFP nylon ("Prepared For Printing") to avoid this problem, probably not possible for a parachute. In some cases unwanted finishes can be removed by washing in very hot water (140°F) with soda ash and detergent, but in many cases these finishes cannot be removed at all.
(Please help support this web site. Thank you.)
Monday, June 08, 2009
What can I use to get my linen fabric slacks white again?
—ADVERTISEMENT—
Rit Color RemoverRit Color Remover can be used to remove dyes that have transferred from other garments
Message: Linen fabric slacks white color, I sent to cleaners twice now they are not the same white color as the matching jacket they look a little off white now. What can I do or use to get my linen fabric slacks white again? Thanks jt
Are they washable? If not, there's nothing you can do at home.
If the linen slacks are washable, you might be able to whiten them in the washing machine by using either Jacquard Color Remover or Rit Color Remover, in hot water. Buy at least two boxes of the Rit Color Remover, and carefully follow the package instructions.
Unfortunately, there's no guarantee you will be able to match the original color of the jacket.
As a general rule, always send all parts of a matching outfit to be cleaned at the same time, so that they will continue to match one another even if their color is slightly changed during the cleaning process.
(Please help support this web site. Thank you.)
Friday, June 05, 2009
Looking for a supplier for for Reactive Blue 5 for research on some dye decolorizing peroxidases
Message: Hello, I was wondering if you know of some suppliers for Reactive Blue 5. I want to use this dye for my research on some dye decolorizing peroxidases.
Colour Index Reactive Blue 5 is a monochlorotriazine anthraquinone dye also known as Cibacron Brilliant Blue BRP, Reactive Blue HGR, Blue K-GR, or Reactive Brilliant Blue K-GRS. Its registry numbers are 16823-51-1 (CAS) and 240-844-9 (EINECS).
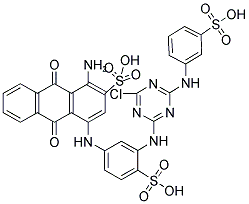
Unfortunately, none of the suppliers I know for small quantities carries this dye. You can't buy it from PRO Chemical & Dye, Dharma Trading Company, Sigma Aldritch, Standard Dye, or Classic Dye. It is not listed on my table of Procion H type dyes.
Many of the dyes formerly made by the Cibacron company are now manufactured by Huntsman Textile Effects under the name Novacron. However, the Huntsman Textile Effects website does not list this particular dye. There is a mention of Novacron Brilliant Blue H-GR on the Town End Leeds website's Novacron page in the UK (scroll down), so perhaps they do still make it. Please contact Huntsman Textile Effects in the US.
The Chemical Book website lists these suppliers for reactive blue 5:
| Ningbo Dynasty Chemical Co., Ltd | info@dynasty-chem.com | CHINA |
| HONEST JOY HOLDINGS LIMITED | sales@honestjoy.cn | USA |
| OCEAN TRADING CORPORATION | otcchem@otcchem.com | INDIA |
| Pylam Products Company, Inc. | sales@pylamdyes.com | USA |
| Advanced Technology & Industrial Co., Ltd. | sales@advtechind.com | CHINA |
Good luck in locating a suitable supplier. (Please help support this web site. Thank you.)
Thursday, June 04, 2009
Can I ombré-dye a dry-clean-only acetate/rayon blend dress?
Message: Hello I would like to know if it is possible to dye a 65% acetate mixed with 35% rayon dry clean only dress? I want to do an ombre effect on it. The dress is cream colored with little rust stain spots. Thanks!
No, sorry, you can't dye anything that is not washable.
In some cases you can machine-wash a dress even if it is marked "dry clean only". If it survives the washing, you can then consider dyeing it.
However, acetate is not a good fiber to try to dye at home. It often gives uneven, splotchy results, and it requires stovetop boiling and special dyes for best results. You can try dyeing it in the washing machine with all-purpose dye, but the results are unlikely to be worth wearing. It's not worth the time, trouble, and expense.
If you want to dye an ombre effect on a dress, I strongly recommend you buy a dyeable white dress blank, such as those that Dharma Trading Company sells. It will give you much better results, and often for a very reasonable price.
Dyeing color gradients is more difficult than other sorts of dyeing. See "dyeing single color with gradual fading effect" and "Questions about ombre / gradation dyeing long piece of fabric...". I do not recommend ombre dyeing for beginning dyers; it's a technique more suitable for people with considerable experience in hand dyeing.
(Please help support this web site. Thank you.)
Wednesday, June 03, 2009
Do you know of a way to dye fringed silk devore scarves without tangling the fringe?
Message: Do you know of a way to dye fringed silk devore scarves without tangling the fringe. HELP! Its taking a long time to untangle them.
Dyeing fringe—or rather, untangling the fringe after dyeing—is such a pain that I personally have sworn never to dye anything with fringe on it again.
One thing that helps is to buy small net lingerie bags, and put the scarves into them for all washing and rinsing. These bags are usually easy to find at a drug store. Gently hand-washing, instead of machine washing, is also a good idea. Hang the fringed scarves to dry; don't dry in the machine.
Using a high quality fabric softener can help some, too. Don't use the grocery store fabric softeners if you have any alternatives. The fabric softeners sold by dye retailers, such as Dharma Trading Company and by PRO Chemical and Dye, are much stronger and more effective and lack the allergenic perfumes.
A lot of dyers use a wide-toothed comb or plastic brush to help untangle the fringe. This is a slow process and requires patience. Some people dip just the fringe into well-diluted hair conditioner before untangling. I've heard that Dharma Trading Company advises untangling the fringe while it is still in the water with the fabric softener or hair conditioner.
Here's a relevant discussion on the Dye Forum: "Reconditioning frazzled fringe".
If any of these tips work particularly well for you, please let me know!
(Please help support this web site. Thank you.)
Tuesday, June 02, 2009
We would like to temporarily dye white doves red and blue for about 30 seconds after release
—ADVERTISEMENTS—
—ADVERTISEMENTS—
Message: Hi, I hope this hasnt been asked before although I saw similar. We would like to temporarily dye white doves red and blue for a 4th of july release. We would need the dye to last for about 30 seconds after release and whatever time it would be to take them to the release site. Of course a dye would last longer and most people want longer but we want shorter!
There is no dye that will completely disappear that quickly.
There are colored chemicals that will lose their color quickly while still remaining present. This could give an effect similar to what you're asking for, but would do nothing particularly good for the birds, since the chemical will still be present.
Another problem is that the disappearing inks are not available in the colors you want, but typically only in a bright violet color. You can find this product in the "disappearing ink markers" sold by fabric stores for temporarily marking fabric. I don't know how safe this product would be for birds.
The speed with which temporary marking colors disappear varies greatly depending on the amount of moisture or humidity present. It would take a considerable amount of trial and error to work out how much to apply. You could apply the color, then, when you wish the color to start disappearing, mist the colored birds with water, which generally makes disappearing ink decolorize quickly.
All in all, this is an interesting idea, but I do not recommend that you pursue it.
Perhaps you should acquire some smoke dye and create a temporary burst of colored smoke, instead. Please note that textile dyes cannot be used to color smoke; you must mail-order "smoke dyes", specifically, such as those sold by Skylighter.com.
(Please help support this web site. Thank you.)
Monday, June 01, 2009
Can I soak the tie dye shirts done with fiber-reactive dye in the soda ash after the dyeing process?
Message: Hi,
Can I soak the tie dye shirts done with fiber-reactive dye in the soda ash after the dyeing process? I didn't put it in before dying.
Yes, you can apply the soda ash after applying the dye. It may make the colors run a bit, but this is far preferable to having them unfixed.
If you've already untied your fabric, put the soda ash solution into a spray bottle and mist the fabric lightly, then wrap in plastic and keep it moist overnight for the dyes to react. If your fabric is still dyed, just pour some soda ash solution over it.
It's good that you used fiber reactive dyes. Soda ash works extremely well for fixing fiber reactive dyes, such as Procion MX dyes. Soda ash should not be used with all-purpose dyes; it will not fix Rit or Tintex dyes. (To fix all-purpose dye, use a mail-order cationic dye fixative such as Retayne or Jacquard iDye Fixative.)
Some tie-dye kits include soda ash as a separate presoak. Others contain it already mixed with the dyes, so the soda ash starts reacting with the dye as soon as you mix them with water.
Also see the FAQ page, "What is soda ash, and what's it for in dyeing?".
(Please help support this web site. Thank you.)

