Chapter 3. Hydroxyl Radical Production in Vitro
Introduction
The protective effects in E. coli of the hydroxyl radical scavengers thiourea
and DMSO in vivo strongly suggest that the agent directly responsible for cell
damage and death as a result of the photodynamic effect is the hydroxyl radical
(OH.).200 In addition, protection by extracellular SOD and catalase in the cell
incubation medium suggest that both O2- and hydrogen peroxide are involved in
photodynamic kill.200 In order to investigate which reactants are necessary for the
production of OH. by dye mediated photooxidation in vivo, direct in vitro assays were
utilized under a variety of experimental conditions. The production of OH. as a result
of reactions by the illuminated dyes requires the presence of an oxidizable
substrate, such as NAD(P)H; other oxidizable substrates of potential in vivo
relevance were also studied for their ability to substitute for NADH in this reaction. In
addition, the ability of various iron chelators to catalyze the Haber-Weiss
production of OH. was assessed, as was the ability of Cu++ to catalyze the reaction. A
number of different dyes were investigated using these assays to demonstrate that
all were capable of OH. production. Two different OH. assays were used, as
described below. These assays are the thiobarbituric acid assay and the salicylate
hydroxylation assay. The thiobarbituric acid assay is more sensitive, both to
interference and to detection of small amounts of OH. production, than the salicylate
assay, while the salicylate assay is more quantitative, yielding better estimates of
the actual amount of OH. produced.
Materials & Methods
OH. production was assayed using two methods: the salicylate assay and the
thiobarbituric acid assay, both according to the methods of Halliwell and
Gutteridge

. All assays were carried out at room temperature; heating of solutions by light sources never increased temperatures over 255C.
Salicylate assay The salicylate assay involves exposing salicylic acid to a
source of OH.. In a total reaction volume of 2.00 ml of phosphate buffer (150 mM
potassium phosphate buffer, pH 7.6), 4 mM sodium salicylate (400 ml of 20 mM in the
same phosphate buffer), dye, oxidizable substrate at a concentration of between 0.1
and 2.0 mM, scavenger if indicated, 100 mM EDTA (40 ml of a 5 mM solution), and
100 mM FeCl3 (40 ml of a 5 mM solution) were combined in a glass tube and exposed
to light. Iron levels used in the in vitro assays are realistic in view of the ability of E.
coli to sequester iron using enterochelin and related
compounds.
The phosphate buffer was treated with Chelex 100 before use to remove as much
contaminating iron as possible, by stirring with Chelex (8 grams per liter of buffer) for
eight hours and then filtering out the Chelex granules with Whatman #1 filter paper.
The light source was two 20 W. fluorescent bulbs, one on either side of the row of
tubes and in direct contact with the tubes; one tube was a GE Warm White bulb,
while the other was a
_____________________________________________________________
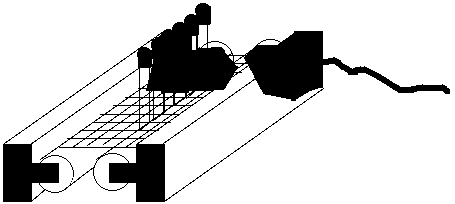
___________________________________________________
Figure 24. Set-up of hydroxyl radical assay experiments. The Warm White bulb on
the left and the Easy-Gro bulb on the right are both in direct contact with the outsides
of the tubes.
Sears Easy-Gro plant grow light bulb (fig. 24). After this incubation, the colorimetric
assay was performed to quantify the presence of 2,3-dihydroxybenzoic acid.
Salicylic acid is specifically hydroxylated by OH. and not by peroxides such as H2O2
or by O2-. The primary hydroxylation product is the ortho diphenyl,
2,3-dihydroxybenzoic acid, which is detected in the assay, although some
hydroxylation in the meta and para positions does occur. Hydrochloric acid (80 ml of
an 11.6 M solution), 0.5 g NaCl, and then 4 ml chilled ether were added to the original
reaction mixture, and vortexed for 30 seconds. Three mls of the upper, ether layer
were then removed to another tube and evaporated until no trace of liquid remained.
The residue was dissolved in 0.25 ml cold distilled water, and trichloroacetic acid
(0.125 ml 10 % (w/v) in 0.5 M HCl), sodium tungstate (0.25 ml 10% (w/v) in H2O), and
sodium nitrite (0.25 ml 0.5% (w/v) in H2O) were added, in that order. The solutions
were left standing for five minutes, and then KOH (0.5 ml of 0.5 M) was added;
exactly sixty seconds after the addition of KOH, the absorbance at 510 nm was
determined. 2,3-dihydroxybenzoate was added to mock reaction mixtures, and the
same extraction procedure followed in constructing the standard curve. With
practice, I obtained higher yields on this assay, mainly through increasing the
amount of vortexing at the step when the residue after ether evaporation was
dissolved in water; unfortunately, this means that numbers cannot be compared
between assays performed at different times, but only within a given experiment,
which usually comprised 18 to 36 assays. Each figure shows the result only of
assays performed at one time. The salicylate assay never yielded a value of zero;
typically, dye alone and oxidizable substrate alone each yielded a small but real
amount of absorbance. In most experiments, therefore, a background value,
generally around 10 to 20% of the total, was subtracted. This value was obtained by
adding the yield of dye alone to the yield of substrate alone and subtracting the value
obtained in the absence of either dye or substrate. Since all of these values were
altered by the presence of different chelators or the use of different experimental
times, however, and since practical considerations limited the total number of
assays that could be done in each experiment, the background value was not known
in certain experiments; these experiments are indicated in their respective figure
legends.
Thiobarbituric acid assay The thiobarbituric acid assay is performed using
deoxyribose as the detector molecule, with a colorimetric assay that detects the
formation of malondialdehyde, a fragmentation product of deoxyribose.
Fragmentation occurs by a decomposition reaction initiated by abstraction of a
hydrogen atom from carbon 4 of the deoxyribose ring by OH.. The reaction is
initiated only by very strong oxidants such as OH., not by O2- or H2O2 alone. 1.5 mM
deoxyribose (200 ml of a 5 mM solution in phosphate buffer), 220 mM FeCl3 (50 ml of a
1 mM solution), dye, oxidizable substrate (0.5 mM reduced NADH unless otherwise
indicated), 220 mM chelator (EDTA, 20 ml of a 5 mM solution, unless otherwise
indicated), and scavenger (if indicated) were combined in a total volume of 680 ml
phosphate buffer (150 mM potassium phosphate buffer, pH 7.6) and exposed to light
for thirty minutes as described for the salicylate assay. Chelator conncentrations
were always kept . Fe3+ concentrations to ensure that all Fe3+ stayed soluble in the
buffer solution. After light exposure, 0.3% thiobarbituric acid (500 ml of a 1% (w/v)
solution in 0.05 M NaOH) and 0.83% trichloroacetic acid (500 ml of a 2.8% (w/v)
solution) were added, the tubes were placed in boiling water for 10 minutes and then
allowed to cool. Under these conditions malondialdehyde combines with
thiobarbituric acid to form a pink chromophore. The absorbances at 532 nm were
determined
on a Gilson spectrophotometer. Malondialdehyde was used to prepare a standard
curve in order to convert absorbance to number of moles of product.
Both assays were tested for specificity in determining OH. by competition
with a variety of known OH. scavengers.
Results
Dyes A number of dyes were surveyed for their ability to mediate OH.
production. In the presence of 1 mm NADH, 100 mm Fe(III), and 100 mm EDTA,
thionin, proflavin, neutral red, fluorescein, toluidine blue o, rose bengal, methylene
blue, and acridine orange all stimulated the production of large amounts of OH.
relative to the sensitivity of the salicylate assay; lucifer yellow and quinacrine
produced very low levels (fig. 25).
Time The OH. production mediated by by azure c and proflavin was studied
as a function of time (fig. 26). Most of the reaction sensitized by either dye was
completed by thirty minutes; thus, subsequent experiments were performed using
thirty minute incubations. As the dye reactions are cyclic, resulting in the
regeneration of the dye in its original form, it is probably the exhaustion of reductants
or of dissolved oxygen that limit OH. production.
Concentrations of dyes Azure c, proflavin, and neutral red generated
increasing amounts of OH. as dye concentration increased (fig. 27). The thiazine
azure c mediated more OH. production at a given concentration than did proflavin,
which in turn was more effective than the phenazine neutral red; the substituted
acridine quinacrine did not produce OH. in the presence of 1 mm NADH. This pattern
of reactivity parallels the relative reactivities of these dyes toward NADH under the
illumination conditions employed, as determined in another study.183
Oxidizable substrates Several potentially physiological substrates were
tested with each of the dyes azure c, proflavin, and rose bengal (fig. 28) . These
substrates were tested at concentrations similar to those at which they occur in vivo,
and under in vivo pH conditions. NADH and NAD(P)H are oxidized with equal
effectiveness by dyes, and all experiments requiring NAD(P)H were carried out with
NADH for the sake of economy. Reduced glutathione and NADH were both effective
substrates for all three dyes. Under the same conditions, cysteine was an effective
substrate for azure c and proflavin, but not for rose bengal; tryptophan and tyrosine
were effective, albeit weaker, substrates for azure c, but failed to produce enough
OH. when reacted with proflavin or rose bengal to be detected by the relatively
insensitive salicylate assay.
Figure 26. Hydroxyl radical production mediated by azure c and proflavin as a
function of time in salicylate assay. Concentrations were: azure c, 1.25 mM;
proflavin, 5 mM; NADH, 1 mM; EDTA, 200 mM; FeCl3, 100 mM. Background values
were not subtracted.
Figure 27. Production of OH. increased as the concentration of dye increased in the
salicylate assay. Other concentrations: NADH, 1 mM; EDTA, 100 mM; FeCl3, 100
mM. Time of light incubation was 30 minutes as described in Materials and Methods.
Azure c, ; proflavin, ; neutral red, ; quinacrine, .
Figure 28. Effectiveness of various substrates in the salicylate assay.
Concentrations were: azure c, 1.25 mM; proflavin, 10 mM; rose bengal, 10 mM;
cysteine, tryptophan, glutathione, tyrosine, and NADH, 1 mM; EDTA, 100 mM;
FeCl3, 100 mM.
While glutathione was not found to be as active as NADH when the salicylate
assay was used to study it, in reactions with the four dyes methylene blue, neutral
red, acridine orange, and fluorescein, it did serve as a substrate for all four of these
dyes (fig. 29). Moreover, glutathione levels within the cytoplasm of E. coli exceed
those of NAD(P)H by 5 to 10-fold.
Glutathione was nearly as active as NADH in a thiobarbituric acid assay (fig.
30); cysteine, methionine, tryptophan, tyrosine, and GMP were also capable of
acting as oxidizable substrates for azure c. Ascorbate appears to have no
effectiveness due to the subtraction of no-dye backgrounds; actually, ascorbate's
effectiveness in the absence of dye was very marked, resulting in an assay value
equivalent to the production of 25 nmoles OH.. Evidently ascorbate was so powerful
as a reductant of Fe-EDTA in the absence of dye that there was no more capacity for
reaction left for the dye to enhance, with amount of detector molecule as the likely
limiting factor. Cysteine was also quite active in the absence of dye, producing 7.0
nmoles of OH.; however, it was even more active in the presence of the dye, as the
figure shows. In the absence of dye, glutathione produced very little positive assay,
equivalent to the production of 0.2 nmole OH.; other low values were: methionine,
0.06; tryptophan, 0.06; tyrosine, 0.05; histidine, 0; GMP, 0.29; and ATP, 0.20. NADH
produced more, as usual, about 2.7 nmoles.
Metals and chelators The catalysis of the dye-mediated production of OH.
was studied to determine metal ions and chelators of in vivo relevance. Two metal
ions, iron(III) and copper (II), were examined. Iron(III) is most effective in catalyzing
the Haber-Weiss reaction when it is chelated by EDTA; however, copper is more
effective when unchelated than when chelated with EDTA (data not shown). As
trace amounts of metals were not removed from the dyes or
other reagents, the treatments probably contained micromolar quantities of
iron,
although the buffer had been treated with Chelex-100 to remove iron. The
effectiveness of EDTA-chelated iron appears to reach a maximum at around 10
mm; at the highest concentration tested, 50 mm, unchelated copper
had not yet reached a maximum, and was about as effective as 2.5 mm iron (fig. 31).
The presence of added iron (50 mm) had no significant effect on the levels of
OH. detection in the absence of chelator, or in the presence of 100 mm deferoxamine,
an iron chelator known to prevent the Haber-Weiss reaction; however, in the
presence of DTPA levels of OH. detection were increased over 3-fold by addition of
iron, while in the presence of EDTA, 50 mm iron increased OH. to an even higher
level, over 31/2-fold to 125 nmoles per assay (fig. 32). Deferoxamine diminished the
detection of OH. only slightly in comparison to the no-chelator reactions.
The chelator DTPA is known to be incapable of chelating iron so to allow it to
catalyse the formation of OH. when the reductant is O2-, as in the Haber-Weiss
reaction, and yet, for most of the dyes studied, it was found to allow the production of
nearly as much OH. as EDTA did (fig. 33); lucifer yellow was an exception, showing
no OH. production in the presence of DTPA. This result is not surprising in light of the
fact that
Winterbourne

has found that paraquat performs in this way, too: evidently the paraquat
monocation radical (and by extension the semireduced dye radical) is a more potent
reductant than is the O2- radical, and therefore substitutes for the latter in the
reduction of the chelated iron. Superoxide is not capable of reducing the
DTPA-chelated iron, which would permit the reduced iron to react with hydrogen
peroxide to produce OH.. However, the excited, reduced dye can reduce the
DTPA-chelated iron.
Figure 32. Chelators with and without iron in the salicylate assay. Concentrations
were: azure c, 1.25 mM; NADH, 1 mM; FeCl3, 100 mM; EDTA, 200 mM; DTPA, 200
mM; deferoxamine, 200 mM.
Table III. Scavenger Effects on Measurement of Hydroxyl
Radical Production by Two Assays.a
_____________________________________________________________
Methylene blue Neutral Red Proflavin Fluorescein
assay: TBA SAL TBA SAL TBA SAL TBA SAL
Dye [ ] (mM): 1.25 1.25 5 50 1.25 10 5 1.25
_____________________________________________________________
Scavenger
None 7.2 59 8.6 79 7.7 57 10.4 52
Urea 6.7 (NA) 6.9 (NA) 4.2 74 9.4 (NA)
Thiourea 0 0 0 (NA) 0.2 0 0 (NA)
Ethanol 0 0 0 (NA) 0.2 0 0 (NA)
Benzoate 1.6 0 1.5 0 4.2 0 1.8 0
DMSO 0 0 0 0 0.3 0 0 11
SOD 5.9 22 6.9 105 6.6 (NA) 7.7 23
Catalase 0 0 0 26 3.5 (NA) 0 16
_____________________________________________________________
aValues are in nmoles of OH.. Dye concentrations were as indicated. Other
concentrations were: NADH, 1 mM; FeCl3, 100 mM; EDTA, 200 mM. NA = data not
available.
The Fenton reaction, which is the reduction of H2O2 to OH., is readily catalyzed by
iron chelated by either EDTA or DTPA. Superoxide, then, serves solely as a source
for hydrogen peroxide in the DTPA-catalyzed reaction. This result also explains
the repeated inability of superoxide dismutase to inhibit the production of OH.
(Table III). In many reactions, SOD appears to increase OH. prod- uction; this may
be due to the fact that SOD actually accelerates the production of hydrogen
peroxide, a necessary substrate for the Fenton reaction.
When a number of potential biological chelators were tested for the ability to
chelate iron in such a way that it would be capable of catalyzing the Haber-Weiss
reaction, several were found to have this activity.
Thiobarbituric acid assays indicated that not only DTPA and EDTA were good
chelators, but also ATP and ADP (fig. 34). Results for citrate, GTP,
2,3-dihydroxybenzoate (2,3-DHB), picolinic acid, 2,4-dipyridyl, oxalic acid, and
pyrophosphate are less definitive, but suggest that these, too, may have some
ability to chelate iron so that it has catalytic activity. Deferoxamine, isocitrate,
phytate, and lactate appear to be without this ability. These compounds were
chosen for their potential biological significance. 2,3-DHB, for example, is a
structural component of the physiological chelator enterochelin found in E. coli;
phytate is a natural chelator found in plant seeds which helps to prevent rancidity.
Oxalic acid is found in many plant
foods.
Salicylate assays confirmed that, in addition to EDTA, ATP, ADP, and citrate were
good chelators(fig. 35A). Succinate, picolinic acid, the antibiotic oxolinic acid, and
the phototoxic antibiotic nalidixic acid, also appeared to be good chelators. In
another salicylate experiment (fig. 35B), phenanthroline and dipyridyl were found to
have some catalytic-chelator activity, but phytate, oxalate, histidine and the
phototoxic chemotherapeutic agent methotrexate were without this activity.
A potential chelator of particularly noteworthy importance is DNA itself. The
multitude of negatively-charged phosphate groups on the outside of the DNA
suggests that it could have chelating abilities. Since DNA serves as both a major
target of hydroxyl-radical produced damage, and actually holds thiazine,
phenazine, and acridine dyes in place through
intercalation,

any chelating activity, however small, could be of enormous in vivo relevance. It was
found in salicylate assays that purified calf thymus DNA promoted OH. production in
the
absence of other chelators (fig. 36 and 37). While the addition of DNA to an
EDTA-containing reaction seemed to reduce the amount of OH. seen, the
substitution of DNA for EDTA gave significantly greater yield of OH. than did no
chelator at all. When increasing concentrations of DNA were studied, it was found
that beginning at concentrations of 12.5 mm of the phosphate groups of the DNA,
OH. production began to increase; at a concentration of 31.25 mm (a DNA
phosphate:dye ratio of 25:1), and above, the DNA and the 50 mm EDTA were similar
in their effects.
All of the dyes were studied in the presence of concentrations of DNA at
which most of the available dye molecules would be intercalated, in order to
ascertain that the results obtained in the absence of DNA would still hold true for
dyes that were intercalated into DNA. Increasing quantities of DNA slightly
decreased the detection of OH. for both the thiobarbituric acid assay (fig. 38) and the
salicylate assay (fig. 39). This was true even for the non-intercalating xanthene dye
fluorescein (fig. 40), suggesting that the reduction in yield was not due to
inaccessibility of intercalated dye. The small reduction in yield observed indicates
that inaccessibility is not a major problem for intercalated dyes. Since DNA actually
reacts with OH., it probably competes with the indicating scavenger for the OH.
which is generated by the dyes.
Scavengers A variety of OH. scavengers was used to confirm that the two
OH. assays were measuring OH. instead of some other reactive species, such as
singlet oxygen (see Table III, above). Thiourea, ethanol, benzoate, and DMSO all
significantly reduced the reaction of OH. with the detector molecules. Urea did not,
as was expected since it is a poor scavenger of the OH.. Catalase had a marked
inhibitory effect on OH. production; superoxide dismutase was partially inhibitory for
methylene blue and fluorescein but actually increased the
Figure 36. DNA as a chelator in the azure c mediated production of OH. as detected
by the salicylate assay. Concentrations were: azure c, 1.25 mM; NADH, 0.5 mM;
EDTA (when present), 50 mM. Light incubation was for ninety minutes. Purified calf
thymus DNA was ethanol-precipitated and washed in ethanol to rid it of EDTA.
Figure 37. Production of OH. mediated by azure c in the presence or absence of
EDTA or DNA. Concentrations were: azure c, 1.25 mM; NADH, 1 mM; FeCl3, 100 mM;
EDTA, 200 mM (when present); DNA, 90 mM (when present).
Figure 38. Production of OH. mediated by DNA-intercalated azure c and methylene
blue, as measured in TBA assay. Concentrations were: methylene blue, 1.25 mM;
azure c, 1.25 mM; NADH, 1 mM; FeCl3, 125 mM; EDTA, 200 mM.
Figure 40. Production of OH. mediated by DNA-intercalated and non-intercalated
methylene blue, neutral red, acridine orange, and fluorescein. Concentrations were:
NADH, 1 mM; FeCl3, 100 mM; EDTA, 200 mM; methylene blue, 1.25 mM; neutral red,
5 mM; acridine orange, 2.5 mM; fluorescein, 5 mM. DNA phosphate:dye molar ratio
was 100:1 for methylene blue, 50:1 for neutral red, 100:1 for acridine orange, and
25:1 for fluorescein. Incubation time for methylene blue was 1/2 hour, as usual, but
was extended to one hour for the other three dyes.
reactivity of neutral red. As discussed above, the semi-reduced neutral red dye
radical probably reduces the chelated iron, substituting for the role of O2- in the
iron-catalyzed Haber-Weiss reaction. Methylene blue, proflavin, and fluorescein
probably also act in this way, although with these dyes both of the pathways of
Fe3+-EDTA reduction (the O2--dependent and the dye.--dependent) probably
make significant contributions, since SOD did show some degree of protection.
Four scavengers, DMSO, benzoate, formate, and ethanol, were assayed
over a wide range of concentrations for their ability to compete with salicylate in
reacting with OH. (fig. 41). The concentrations at which they competed equally with
4 mM salicylate, resulting in 50% inhibition of reaction, were approximately 4 mM for
benzoate, 6 mM for DMSO, 11 mM for formate, and 40 mM for ethanol. This ordering
is the same as would be predicted from their respective rate constants with OH.,
except for DMSO and benzoate, which are very close in either case. The rate
constants for the reactions of these scavengers with OH., as given in Table I, above,
are DMSO, 7.0 x 109; benzoate, 6.0 x 109; formate, 2.8 x 109; and ethanol, 1.5 x 109.92
In view of its historically frequent use as a singlet oxygen scavenger, azide
was tested for its ability to scavenge OH. in the salicylate assay (fig. 42). Sodium
azide was found to be highly effective as a scavenger in this assay, which is not
surprising since it has been shown that sodium azide reacts at nearly diffusion
limited rates with OH. as determined in pulse radiolytic studies.92
Addition of hydrogen peroxide Hydrogen peroxide was added to the dye
mediated photooxidation reactions to assess the role of substrate limitation in OH.
production. The amount of OH. detected was increased dramatically by 100 mm
H2O2 for the thiazine dyes azure c and thionin, the acridines proflavin
Figure 42. Azide as an OH. scavenger in the salicylate assay. Concentrations were:
azure c, 1.25 mM; fluorescein, 5 mM; proflavin, 10 mM; neutral red, 50 mM; NADH, 0.5
mM; FeCl3, 100 mM; EDTA, 200 mM; DMSO, 350 mM; sodium azide, 25 mM.
and acridine orange, the xanthene fluorescein, the phenazine neutral red, and the
napthalimide lucifer yellow (fig. 43). Catalase and the OH. scavengers thiourea,
benzoate, and DMSO were able to inhibit both the normal amount of reaction and
the amount of excess reaction produced by the presence of hydrogen peroxide
(fig. 44). This is consistent with the hypothesis that the dyes are producing O2-,
which dismutes to form hydrogen peroxide and ultimately OH., with semi-reduced
dyes substituting for O2- in the reduction of chelated iron(III). It is also consistant with
the observation that SOD increases the amount of OH. detected in the thiobarbituric
acid assay reaction sensitized by neutral red.
Discussion
In this chapter, data have been presented which indicate that all of the dyes
tested except for quinacrine and lucifer yellow, including the thiazines thionin, azure
c, and methylene blue, the acridines proflavin and acridine orange, the xanthenes
fluorescein and rose bengal, and the phenazine neutral red, produce significant
quantities of hydroxyl radicals. The amount of OH. produced was dependent on time
and dye concentration. Oxidizable substrates were required for the production of
large amounts of hydroxyl radicals, although some hydroxyl radical production was
detected even in their absence; effective substrates for the dye azure c included
NADH, glutathione, cysteine, tryptophan, tyrosine, methionine, and GMP. Metal
ions were required as catalysts of OH. production. Both copper and chelated iron
proved to be effective catalysts. Chelators that could enable iron to act as a catalyst
for OH. production included EDTA and DTPA as well as the biologically significant
compounds ATP, ADP, citrate, GTP, oxalic acid, 2,3-DHB, succinate
pyrophosphate, and purified large
Figure 44. Hydroxyl radical scavengers prevent enhancement of OH. production
sensitized by azure c by hydrogen peroxide as assayed with salicylate.
Concentrations were: azure c, 1.25 mM; NADH, 1 mM; FeCl3, 100 mM; EDTA, 200
mM; H2O2, 20 mM; catalase, 1500 units/ml; thiourea, 25 mM; benzoate, 25 mM;
DMSO, 350 mM; urea, 25 mM. Light incubation was for only fifteen minutes.
molecular weight calf thymus DNA. The H2O2 scavenger catalase prevented OH.
production, showing that H2O2 is required for the dye-mediated production of OH..
However, the O2- scavenger SOD did not prevent OH. production, indicating that
the semireduced dye can substitute for O2- in reducing the chelated iron, as is also
indicated by the fact that DTPA-chelated iron acts as a catalyst for the
dye-mediated production of OH., although superoxide cannot reduce
DTPA-chelated iron. Hydroxyl radical scavengers including thiourea, ethanol,
benzoate, azide, and DMSO prevented the reaction of OH. with the detector
molecules used in the assays, confirming the specificity of the assays.
Supplementation of the reactions with additional H2O2 increased the production of
OH.; with semi-reduced dye reducing the chelated iron, the Fenton reaction yield is
increased.
In Chapter Two, we saw that the illuminated dyes were able to oxidize NADH
and produce O2- in vitro; this chapter shows that they also produce OH. in vitro, and
extends our knowledge to what the requirements for OH. production are. All of the
requirements outlined in this chapter, including those for oxidizable substrate, iron,
and chelator, are met by the contents of normal cells, and are therefore of potential in
vivo significance.
next page