« 2008 January | Main | 2007 November »
Monday, December 31, 2007
I'm wondering if you could explain the chemistry behind why cotton can't be dyed at an acidic pH.
Saturday, December 29, 2007
Can copper sulphate, ferric chloride, and potassium permanganate be used as mordants?
Friday, December 28, 2007
What dyes can I use to dye mohair fabric for teddy bears?
Thursday, December 27, 2007
I have a ski coat, 49% wool, 26% polyester, 13% nylon, 12% polyurethane, that is too funky in colour for me to wear. Can I dye it?
Wednesday, December 26, 2007
Can I use Jacquard silk colors (green label) to dye my wool roving as well or do I have to use another type of dye?
Tuesday, December 25, 2007
solving problems with immersion dyeing to a single solid shade
Thursday, December 20, 2007
Is there any way to dye with cold water?
Wednesday, December 19, 2007
what to use to dye soft white polyurethane foam
Friday, December 07, 2007
problems in dyeing feathers
Thursday, December 06, 2007
Can I dye my pants black to hide oily food stains, or will the stains come thru the new dye?
Tuesday, December 04, 2007
Would you have any information on potential hue shifts of Lanaset dyes due to fading?
Monday, December 03, 2007
Do you have any tips for dyeing fur?
Sunday, December 02, 2007
Can I substitute guar gum for alginate in screen printing with Procion MX type dyes?
Saturday, December 01, 2007
thermochromic pigment changes color when warm, and changes back again when cool
I'm wondering if you could explain the chemistry behind why cotton can't be dyed at an acidic pH.
Name: Lulu
Message: Hello, I'm wondering if you could explain the chemistry behind why cotton can't be dyed at an acidic pH. I did an experiment at school where I used 15 mL of HCl to dye a 100% cotton cloth using the Procion MX dye (fuschia) and the colour absorbed by the cotton appeared the same as the one with a pH of 8. The concentration of HCl was 1 mol/L. Thanks
At a pH of 8, I see quite a bit of reaction between Procion MX dye and the fiber, though much less than at a pH of 9 or 10, but I see essentially none at pH 7 and below. The small amount of HCl (hydrochloric acid) that you used probably produced a pH just below 7, but if your water supply is very alkaline the final pH may still have been above 7. (You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.
(You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.
Cotton fibers are made of long molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.
molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.
In the picture above, the carbons are shown with the letter "C", but in most chemical drawings, there are so many carbons that they are not drawn at all; wherever two lines meet at an angle, if there is no other letter written there, you can assume that that indicates that there is a carbon atom at the junction of two lines. Here is a drawing of a section of a cellulose molecule, showing how the glucose units are connected together to form the cellulose chain: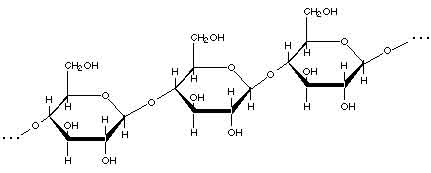
(The darker lines are supposed to make the molecule look more three-dimensional; you can ignore them for now.)
The way that cellulose reacts with fiber reactive dye, such as Procion MX dye, is shown in the drawing below, from the May 19, 2005 posting in this blog, "Chemical reaction for a dichlorotriazine dye with cellulose". The large molecule shown is the dye molecule, while the cellulose chain is represented by the word "cellulose", in this drawing:
Before the cellulose can react, it must first become activated by losing a hydrogen ion to become what is called a cellulosate anion, with a negative charge. After the cellulose has lost its hydrogen ion, it can attack the carbon adjacent to one of the chlorines in the dye molecule. (The chlorine makes that carbon atom more electronegative than any of the carbons that do not have a chlorine atom attached to them, so it is somehow more vulnerable to attack.) In the presence of a high pH, such as is provided by soda ash or sodium hydroxide, the cellulose readily loses that hydrogen in order to become a cellulosate anion. Think of it this way: at a pH above neutral, there are, in effect, extra hydroxyl ions (OH) floating around in the water. With more free hydroxyls, that hydrogen on the cellulose is more easily stolen away to make a new water molecule, with two hydrogens and one oxygen. However, when you add acid, such as the HCl you used, this reduces the number of available OHs floating around in the water in your dyebath.
After the cellulose is ionized by the loss of that hydrogen atom, it readily reacts with the fiber reactive dye. The dye shown in the drawing above is a dichlorozine or Procion MX dye, red MX-5B, also known as Colour Index reactive red 2. The reaction is similar for other fiber reactive dyes; for example, see the drawing in the posting in this blog for February 21, 2006. You can compare the chemical structure of your fuchsia Procion MX dye (which is red MX-8B, or reactive red 11) on my page "What is the chemical structure of Procion MX dye?", at http://www.pburch.net/dyeing/FAQ/structure.shtml . The two dyes are very similar in structure and in function.
However, in contrast, the "reaction" between a different class of dye, direct dye, with cotton is completely different. Direct dye is the fast-fading dye found in all-purpose dyes, such as Rit dye or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.
or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.
(Please help support this web site. Thank you.)
Message: Hello, I'm wondering if you could explain the chemistry behind why cotton can't be dyed at an acidic pH. I did an experiment at school where I used 15 mL of HCl to dye a 100% cotton cloth using the Procion MX dye (fuschia) and the colour absorbed by the cotton appeared the same as the one with a pH of 8. The concentration of HCl was 1 mol/L. Thanks
At a pH of 8, I see quite a bit of reaction between Procion MX dye and the fiber, though much less than at a pH of 9 or 10, but I see essentially none at pH 7 and below. The small amount of HCl (hydrochloric acid) that you used probably produced a pH just below 7, but if your water supply is very alkaline the final pH may still have been above 7.
 (You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.
(You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.Cotton fibers are made of long
 molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.
molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.In the picture above, the carbons are shown with the letter "C", but in most chemical drawings, there are so many carbons that they are not drawn at all; wherever two lines meet at an angle, if there is no other letter written there, you can assume that that indicates that there is a carbon atom at the junction of two lines. Here is a drawing of a section of a cellulose molecule, showing how the glucose units are connected together to form the cellulose chain:

(The darker lines are supposed to make the molecule look more three-dimensional; you can ignore them for now.)
The way that cellulose reacts with fiber reactive dye, such as Procion MX dye, is shown in the drawing below, from the May 19, 2005 posting in this blog, "Chemical reaction for a dichlorotriazine dye with cellulose". The large molecule shown is the dye molecule, while the cellulose chain is represented by the word "cellulose", in this drawing:

Before the cellulose can react, it must first become activated by losing a hydrogen ion to become what is called a cellulosate anion, with a negative charge. After the cellulose has lost its hydrogen ion, it can attack the carbon adjacent to one of the chlorines in the dye molecule. (The chlorine makes that carbon atom more electronegative than any of the carbons that do not have a chlorine atom attached to them, so it is somehow more vulnerable to attack.) In the presence of a high pH, such as is provided by soda ash or sodium hydroxide, the cellulose readily loses that hydrogen in order to become a cellulosate anion. Think of it this way: at a pH above neutral, there are, in effect, extra hydroxyl ions (OH) floating around in the water. With more free hydroxyls, that hydrogen on the cellulose is more easily stolen away to make a new water molecule, with two hydrogens and one oxygen. However, when you add acid, such as the HCl you used, this reduces the number of available OHs floating around in the water in your dyebath.
After the cellulose is ionized by the loss of that hydrogen atom, it readily reacts with the fiber reactive dye. The dye shown in the drawing above is a dichlorozine or Procion MX dye, red MX-5B, also known as Colour Index reactive red 2. The reaction is similar for other fiber reactive dyes; for example, see the drawing in the posting in this blog for February 21, 2006. You can compare the chemical structure of your fuchsia Procion MX dye (which is red MX-8B, or reactive red 11) on my page "What is the chemical structure of Procion MX dye?", at http://www.pburch.net/dyeing/FAQ/structure.shtml . The two dyes are very similar in structure and in function.
However, in contrast, the "reaction" between a different class of dye, direct dye, with cotton is completely different. Direct dye is the fast-fading dye found in all-purpose dyes, such as Rit dye
 or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.
or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.(Please help support this web site. Thank you.)
Saturday, December 29, 2007
Can copper sulphate, ferric chloride, and potassium permanganate be used as mordants?
Name: azkhan
Message: I want to know whether copper sulphate, ferric chloride, and potassium permagnate can be used as mordants or not? If yes, then kindly provide some information about them. Can you compare natural and synthetic dyes?
Potassium permanganate is not used as a mordant for hand dyeing. It is used industrially to discharge
(bleach out) indigo denim, but it is not recommended for this use at home
because it is very toxic. It is also a serious explosion hazard if you allow a
solution of potassium permanganate to dry up. See this discussion on the
Dye Forum for more information: "Discharge Indigo with
Potassium Permanganate".
is not used as a mordant for hand dyeing. It is used industrially to discharge
(bleach out) indigo denim, but it is not recommended for this use at home
because it is very toxic. It is also a serious explosion hazard if you allow a
solution of potassium permanganate to dry up. See this discussion on the
Dye Forum for more information: "Discharge Indigo with
Potassium Permanganate".
You may be thinking of a very different potassium compound which has, ill-advisedly, been used as a mordant with natural dyes. Potassium dichromate
is a known human carcinogen. Chromium is commonly available in two forms, the
trivalent form and the hexavalent form. Potassium dichromate is in the
hexavalent form. Hexavalent chromium has been responsible for many serious
injuries and deaths due to industrial exposure. You should not bring this
substance into your home, though you could use it in a properly equipped
laboratory. It is very possibly illegal in your community for you to dispose of
it in your trash or as wastewater. In contrast, chrome-complexed premetallized
dyes such as some of the Lanaset dyes contain the much less dangerous trivalent
form of chromium, and at much lower concentrations. (See "What
does it mean when a dye is premetalized with chromium or some other heavy
metal? What are the risks of exposure from using a dye that is premetalized with
chromium?".)
Potassium dichromate
is a known human carcinogen. Chromium is commonly available in two forms, the
trivalent form and the hexavalent form. Potassium dichromate is in the
hexavalent form. Hexavalent chromium has been responsible for many serious
injuries and deaths due to industrial exposure. You should not bring this
substance into your home, though you could use it in a properly equipped
laboratory. It is very possibly illegal in your community for you to dispose of
it in your trash or as wastewater. In contrast, chrome-complexed premetallized
dyes such as some of the Lanaset dyes contain the much less dangerous trivalent
form of chromium, and at much lower concentrations. (See "What
does it mean when a dye is premetalized with chromium or some other heavy
metal? What are the risks of exposure from using a dye that is premetalized with
chromium?".)
Copper sulfate is also known as blue vitriol. It is used with logwood in dyeing blue, and
with cutch, improving lightfastness while imparting a greenish
tinge. Copper sulfate is poisonous and can be deadly when swallowed,
and can also be hazardous to the environment, so use it with care and dispose of
it correctly. Here is a link to a good article on the toxicity
of copper sulfate.
is also known as blue vitriol. It is used with logwood in dyeing blue, and
with cutch, improving lightfastness while imparting a greenish
tinge. Copper sulfate is poisonous and can be deadly when swallowed,
and can also be hazardous to the environment, so use it with care and dispose of
it correctly. Here is a link to a good article on the toxicity
of copper sulfate.
Ferric chloride can be used as a mordant almost interchangeably with ferrous sulfate, but
is considered superior for mordanting silk. Iron works to dull down colors; iron
contamination in alum will ruin bright reds. Iron and tannins combined with
logwood makes a good black. Ferric chloride is less toxic than copper
sulfate, but can be deadly when swallowed, so, as with all mordants and
most dyes, you must take appropriate safety precautions, and keep it well away
from children. The Label Hazard Warning required for ferric chloride is as
follows: "DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. HARMFUL IF
SWALLOWED OR INHALED. AFFECTS THE LIVER." Here is a link to an MSDS for ferric
chloride.
but
is considered superior for mordanting silk. Iron works to dull down colors; iron
contamination in alum will ruin bright reds. Iron and tannins combined with
logwood makes a good black. Ferric chloride is less toxic than copper
sulfate, but can be deadly when swallowed, so, as with all mordants and
most dyes, you must take appropriate safety precautions, and keep it well away
from children. The Label Hazard Warning required for ferric chloride is as
follows: "DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. HARMFUL IF
SWALLOWED OR INHALED. AFFECTS THE LIVER." Here is a link to an MSDS for ferric
chloride.
The only really safe mordants for natural dyes are alum and tannin. Of course they are not safe to swallow,
but they are not deadly. Beginning dyers should not use copper sulfate or ferric
chloride unless they have been trained in the safe use of toxic chemicals; even
experienced dyers should not use potassium dichromate in their homes, where
inadvertent exposures may contaminate living areas.
for natural dyes are alum and tannin. Of course they are not safe to swallow,
but they are not deadly. Beginning dyers should not use copper sulfate or ferric
chloride unless they have been trained in the safe use of toxic chemicals; even
experienced dyers should not use potassium dichromate in their homes, where
inadvertent exposures may contaminate living areas.
For more discussion comparing natural with synthetic dyes, see the following pages:
• "About Natural Dyes"
• "Aren't natural dyes safer than synthetic dyes?"
• "Natural Dyes Are Not Superior to Synthetic Dyes"
• "Fixing natural dyes from walnuts, goldenrod, sassafras and poke weed in
cotton - do I use urea or soda ash?"
• "A Science Fair Project on Artificial Dyes Versus Natural Dyes "
• "What's the difference between mordants and other chemical assistants used in dyeing?"
• "Natural Dyes", on the Dye Forum
(Please help support this web site. Thank you.)
Message: I want to know whether copper sulphate, ferric chloride, and potassium permagnate can be used as mordants or not? If yes, then kindly provide some information about them. Can you compare natural and synthetic dyes?
Potassium permanganate
 is not used as a mordant for hand dyeing. It is used industrially to discharge
(bleach out) indigo denim, but it is not recommended for this use at home
because it is very toxic. It is also a serious explosion hazard if you allow a
solution of potassium permanganate to dry up. See this discussion on the
Dye Forum for more information: "Discharge Indigo with
Potassium Permanganate".
is not used as a mordant for hand dyeing. It is used industrially to discharge
(bleach out) indigo denim, but it is not recommended for this use at home
because it is very toxic. It is also a serious explosion hazard if you allow a
solution of potassium permanganate to dry up. See this discussion on the
Dye Forum for more information: "Discharge Indigo with
Potassium Permanganate".You may be thinking of a very different potassium compound which has, ill-advisedly, been used as a mordant with natural dyes.
 Potassium dichromate
is a known human carcinogen. Chromium is commonly available in two forms, the
trivalent form and the hexavalent form. Potassium dichromate is in the
hexavalent form. Hexavalent chromium has been responsible for many serious
injuries and deaths due to industrial exposure. You should not bring this
substance into your home, though you could use it in a properly equipped
laboratory. It is very possibly illegal in your community for you to dispose of
it in your trash or as wastewater. In contrast, chrome-complexed premetallized
dyes such as some of the Lanaset dyes contain the much less dangerous trivalent
form of chromium, and at much lower concentrations. (See "What
does it mean when a dye is premetalized with chromium or some other heavy
metal? What are the risks of exposure from using a dye that is premetalized with
chromium?".)
Potassium dichromate
is a known human carcinogen. Chromium is commonly available in two forms, the
trivalent form and the hexavalent form. Potassium dichromate is in the
hexavalent form. Hexavalent chromium has been responsible for many serious
injuries and deaths due to industrial exposure. You should not bring this
substance into your home, though you could use it in a properly equipped
laboratory. It is very possibly illegal in your community for you to dispose of
it in your trash or as wastewater. In contrast, chrome-complexed premetallized
dyes such as some of the Lanaset dyes contain the much less dangerous trivalent
form of chromium, and at much lower concentrations. (See "What
does it mean when a dye is premetalized with chromium or some other heavy
metal? What are the risks of exposure from using a dye that is premetalized with
chromium?".)Copper sulfate
 is also known as blue vitriol. It is used with logwood in dyeing blue, and
with cutch, improving lightfastness while imparting a greenish
tinge. Copper sulfate is poisonous and can be deadly when swallowed,
and can also be hazardous to the environment, so use it with care and dispose of
it correctly. Here is a link to a good article on the toxicity
of copper sulfate.
is also known as blue vitriol. It is used with logwood in dyeing blue, and
with cutch, improving lightfastness while imparting a greenish
tinge. Copper sulfate is poisonous and can be deadly when swallowed,
and can also be hazardous to the environment, so use it with care and dispose of
it correctly. Here is a link to a good article on the toxicity
of copper sulfate.Ferric chloride can be used as a mordant almost interchangeably with ferrous sulfate,
 but
is considered superior for mordanting silk. Iron works to dull down colors; iron
contamination in alum will ruin bright reds. Iron and tannins combined with
logwood makes a good black. Ferric chloride is less toxic than copper
sulfate, but can be deadly when swallowed, so, as with all mordants and
most dyes, you must take appropriate safety precautions, and keep it well away
from children. The Label Hazard Warning required for ferric chloride is as
follows: "DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. HARMFUL IF
SWALLOWED OR INHALED. AFFECTS THE LIVER." Here is a link to an MSDS for ferric
chloride.
but
is considered superior for mordanting silk. Iron works to dull down colors; iron
contamination in alum will ruin bright reds. Iron and tannins combined with
logwood makes a good black. Ferric chloride is less toxic than copper
sulfate, but can be deadly when swallowed, so, as with all mordants and
most dyes, you must take appropriate safety precautions, and keep it well away
from children. The Label Hazard Warning required for ferric chloride is as
follows: "DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. HARMFUL IF
SWALLOWED OR INHALED. AFFECTS THE LIVER." Here is a link to an MSDS for ferric
chloride. The only really safe mordants
 for natural dyes are alum and tannin. Of course they are not safe to swallow,
but they are not deadly. Beginning dyers should not use copper sulfate or ferric
chloride unless they have been trained in the safe use of toxic chemicals; even
experienced dyers should not use potassium dichromate in their homes, where
inadvertent exposures may contaminate living areas.
for natural dyes are alum and tannin. Of course they are not safe to swallow,
but they are not deadly. Beginning dyers should not use copper sulfate or ferric
chloride unless they have been trained in the safe use of toxic chemicals; even
experienced dyers should not use potassium dichromate in their homes, where
inadvertent exposures may contaminate living areas.For more discussion comparing natural with synthetic dyes, see the following pages:
• "About Natural Dyes"
• "Aren't natural dyes safer than synthetic dyes?"
• "Natural Dyes Are Not Superior to Synthetic Dyes"
• "Fixing natural dyes from walnuts, goldenrod, sassafras and poke weed in
cotton - do I use urea or soda ash?"
• "A Science Fair Project on Artificial Dyes Versus Natural Dyes "
• "What's the difference between mordants and other chemical assistants used in dyeing?"
• "Natural Dyes", on the Dye Forum
(Please help support this web site. Thank you.)
Friday, December 28, 2007
What dyes can I use to dye mohair fabric for teddy bears?
Name: Christine
Message: Your website is full of useful information. I know I want to dye mohair "Tom" material from LouBear. Which product can I purchase? Can we purchase from you? Which section I can directly learn about the Mohair dye?
Mohair is a natural protein fiber that can be dyed with the same dyes that work on wool. It takes dyes beautifully. It should not be boiled, but must be heated to a high temperature that is below boiling for the dye to take; this temperature should be maintained for at least half an hour or an hour to give the dye plenty of time to bond to the fiber. A good temperature range for dyeing mohair is 145°F to 180°F (63°C to 82°C).
The world's largest online discount art & craft supply store!

For a single smooth solid shade, you should use a relatively large amount of water in a cooking pot, enough that your mohair fabric can move freely as you stir it in the dyebath. For more interesting variegated colors, you can stuff the fabric into quart-sized glass jars, adding the dye ingredients and a smaller amount of water to each jar with the fabric, and place these into a pot of water which you maintain at the desired temperature level. Use a thermometer to make sure your water is at the correct temperature for dyeing. If you use dyes directly in a cooking pot, do not use it again for food, ever, unless the dye you choose to use is food coloring, which is safe for use in food preparation utensils. Food coloring, such as that found in unsweetened artificially flavored drink mix powder, works surprisingly well as a dye for mohair, but commercial acid dyes will work even better.
If the fur of the mohair clumps up into separate locks when it is wet with dye, you will need to physically open the clumps so that the dye can penetrate evenly, as otherwise you will obtain areas that are less intensely dyed, or entirely undyed. Be sure to wear gloves when contacting the dye. You do not want to get dye onto your hands unnecessarily.
The most washfast (resistant to fading when laundered) dyes for mohair include the Remazol fiber reactive dyes, the reactive and metal complex dyes in the Lanaset range of dyes, and other metal complex dyes (also known as premetalized or premetallised dyes). You can use Jacquard Acid dyes, ProChem's Washfast Acid Dyes, Lanaset dyes from ProChem in the US or KraftKolour in Australia, or any other sort of acid dye. You can also use an all-purpose dye, such as Rit dye or Tintex Hot Water Dye, but these will be less resistant to fading when washed. See my page of "Sources for Dyeing Supplies Around the World", for good companies from which to order dyes intended for use on wool and mohair. Follow the manufacturer's instructions for dyeing wool, except for using a lower temperature for your dyebath. You will need to use a mild acid as a helper chemical for your dye, such as Albegal SET for Lanaset dyes (sold by the same companies that sell the Lanaset dyes), or citric acid or ordinary white vinegar for other acid dyes (including food coloring).
(Please help support this web site. Thank you.)
Message: Your website is full of useful information. I know I want to dye mohair "Tom" material from LouBear. Which product can I purchase? Can we purchase from you? Which section I can directly learn about the Mohair dye?
Mohair is a natural protein fiber that can be dyed with the same dyes that work on wool. It takes dyes beautifully. It should not be boiled, but must be heated to a high temperature that is below boiling for the dye to take; this temperature should be maintained for at least half an hour or an hour to give the dye plenty of time to bond to the fiber. A good temperature range for dyeing mohair is 145°F to 180°F (63°C to 82°C).
—ADVERTISEMENT—
Buy Jacquard Acid Dyes from MisterArt.comThe world's largest online discount art & craft supply store!
Jacquard Acid Dyes are concentrated, powdered, hot water dyes that produce the most vibrant possible results on protein fibers including mohair, silk, wool, cashmere, alpaca, feathers, and most nylons.
For a single smooth solid shade, you should use a relatively large amount of water in a cooking pot, enough that your mohair fabric can move freely as you stir it in the dyebath. For more interesting variegated colors, you can stuff the fabric into quart-sized glass jars, adding the dye ingredients and a smaller amount of water to each jar with the fabric, and place these into a pot of water which you maintain at the desired temperature level. Use a thermometer to make sure your water is at the correct temperature for dyeing. If you use dyes directly in a cooking pot, do not use it again for food, ever, unless the dye you choose to use is food coloring, which is safe for use in food preparation utensils. Food coloring, such as that found in unsweetened artificially flavored drink mix powder, works surprisingly well as a dye for mohair, but commercial acid dyes will work even better.
If the fur of the mohair clumps up into separate locks when it is wet with dye, you will need to physically open the clumps so that the dye can penetrate evenly, as otherwise you will obtain areas that are less intensely dyed, or entirely undyed. Be sure to wear gloves when contacting the dye. You do not want to get dye onto your hands unnecessarily.
The most washfast (resistant to fading when laundered) dyes for mohair include the Remazol fiber reactive dyes, the reactive and metal complex dyes in the Lanaset range of dyes, and other metal complex dyes (also known as premetalized or premetallised dyes). You can use Jacquard Acid dyes, ProChem's Washfast Acid Dyes, Lanaset dyes from ProChem in the US or KraftKolour in Australia, or any other sort of acid dye. You can also use an all-purpose dye, such as Rit dye or Tintex Hot Water Dye, but these will be less resistant to fading when washed. See my page of "Sources for Dyeing Supplies Around the World", for good companies from which to order dyes intended for use on wool and mohair. Follow the manufacturer's instructions for dyeing wool, except for using a lower temperature for your dyebath. You will need to use a mild acid as a helper chemical for your dye, such as Albegal SET for Lanaset dyes (sold by the same companies that sell the Lanaset dyes), or citric acid or ordinary white vinegar for other acid dyes (including food coloring).
(Please help support this web site. Thank you.)
Thursday, December 27, 2007
I have a ski coat, 49% wool, 26% polyester, 13% nylon, 12% polyurethane, that is too funky in colour for me to wear. Can I dye it?
Name: Mark
Message: I have a ski coat that is too funky in colour for me to wear. It is made of 49% wool, 26% polyester, 13% nylon, 12% polyurethane. I am not looking for perfection just the ability to wear a $400.00 coat. What would be the best compromise. Thanks
The world's largest online discount art & craft supply store!

Is the ski jacket coated to be water-repellent? If so, you cannot dye it. Water-repellent means dye-repellent.
If the coat quickly absorbs water that is dropped on it, rather than causing it to bead up or run off, then it might be possible to dye it. A material that easily absorbs water would make a poor ski coat, though, so this seems unlikely.
The polyester will not accept any dye except when boiled for an hour with a special kind of dye called disperse dye. Forget about trying to dye the 26% of the fabric that is polyester, and concentrate on the rest. This means that you can get, at most, only 74% of the full intensity of any dye you use, but that can work out okay.
Wool, nylon, and polyurethane (spandex is made of polyurethane) can all be dyed at a temperature of 160°F with 1:2 metal complex dyes in the Lanaset series of dyes, or (with lower resistance to washing out) many other acid dyes. Unfortunately, the different fibers have different affinities for these dyes and will therefore dye to different shades when dyed in the same bath; in order to dye a solid color, you would have to use chemical reserve agents which are not available to the home dyer.

A very large drawback to using a metal complex dye or an acid dye to change the color of your coat is that it would require buying a very large cooking pot, minimum of five gallons, large enough to allow the coat to move freely in the dye water; the pot should not be made of aluminum, or if made of enamel must not be chipped on the inside, and it should never again be used for food after you have used it for dye. This one detail in itself probably dooms your project. Is it worth spending the money to buy such a large pot? If you are not going to be dyeing anything else, it would make far more sense to forget about dyeing this coat, sell the coat in a local consignment shop (or on eBay) and buy another coat; perhaps the consignment shop will have one more to your liking, at a price that would be less than the cost of a new coat.
(Please help support this web site. Thank you.)
Message: I have a ski coat that is too funky in colour for me to wear. It is made of 49% wool, 26% polyester, 13% nylon, 12% polyurethane. I am not looking for perfection just the ability to wear a $400.00 coat. What would be the best compromise. Thanks
—ADVERTISEMENT—
Buy Jacquard Acid Dyes from MisterArt.comThe world's largest online discount art & craft supply store!
Jacquard Acid Dyes are concentrated, powdered, hot water dyes that produce the most vibrant possible results on protein fibers including silk, wool, cashmere, alpaca, feathers, and most nylons.
Is the ski jacket coated to be water-repellent? If so, you cannot dye it. Water-repellent means dye-repellent.
If the coat quickly absorbs water that is dropped on it, rather than causing it to bead up or run off, then it might be possible to dye it. A material that easily absorbs water would make a poor ski coat, though, so this seems unlikely.
The polyester will not accept any dye except when boiled for an hour with a special kind of dye called disperse dye. Forget about trying to dye the 26% of the fabric that is polyester, and concentrate on the rest. This means that you can get, at most, only 74% of the full intensity of any dye you use, but that can work out okay.
Wool, nylon, and polyurethane (spandex is made of polyurethane) can all be dyed at a temperature of 160°F with 1:2 metal complex dyes in the Lanaset series of dyes, or (with lower resistance to washing out) many other acid dyes. Unfortunately, the different fibers have different affinities for these dyes and will therefore dye to different shades when dyed in the same bath; in order to dye a solid color, you would have to use chemical reserve agents which are not available to the home dyer.

A very large drawback to using a metal complex dye or an acid dye to change the color of your coat is that it would require buying a very large cooking pot, minimum of five gallons, large enough to allow the coat to move freely in the dye water; the pot should not be made of aluminum, or if made of enamel must not be chipped on the inside, and it should never again be used for food after you have used it for dye. This one detail in itself probably dooms your project. Is it worth spending the money to buy such a large pot? If you are not going to be dyeing anything else, it would make far more sense to forget about dyeing this coat, sell the coat in a local consignment shop (or on eBay) and buy another coat; perhaps the consignment shop will have one more to your liking, at a price that would be less than the cost of a new coat.
(Please help support this web site. Thank you.)
Wednesday, December 26, 2007
Can I use Jacquard silk colors (green label) to dye my wool roving as well or do I have to use another type of dye?
Name: karen
Message: My question is...I have been using Jacquard silk colors (green label) to dye my silk fabric and I would like to know if I can use that dye to dye my wool roving as well or do I have to use another type of dye?
The world's largest online discount art & craft supply store!


Yes, this should work. Jacquard Green Label Silk Colors are Remazol-type fiber reactive dyes, with acid and some other chemicals added to make it easier to use without steaming. Since wool is a protein fiber like silk, I think that you should be able to use it on wool in just the same way that you do on silk, either with steaming to set the dye, or with the chemical fixative called Jacquard Permanent Dyeset Concentrate. The Permanent Dyeset Concentrate produce slightly less intense colors than those that steaming can produce with the same dye, but is more convenient to use.
For wool, I would prefer to use Jacquard Red Label Silk Colors, or the less expensive (but more concentrated) Liquid Reactive Dyes sold by PRO Chemical & Dye. These all contain the same type of dye. The Jacquard Red Label dyes must usually be special-ordered, from the same places that stock Jacquard Green Label Silk Colors; it is often easier, and always more economical, to order ProChem's version. The advantage of Jacquard Red Label Silk Colors, as compared to the Green Label Silk Colors, is that the Red Label dye is twice as strong as the Green Label dye, and does not contain the chemicals which make Green Label Silk Colors unsuitable for immersion dyeing on the stovetop, due to the fumes that are produced. (Unlike the Red Label Silk Colors, you cannot use Green Label Silk Colors on cotton and other cellulose-containing fibers, because the Green Label dyes contain acid, while cotton requires a high pH to react with these dyes.) Red Label Silk Colors are little besides pure dye, dissolved in water, without the additives of the Green Label dyes; they can be used on any natural fiber. ProChem's Liquid Reactive Remazol dyes are at least four times as concentrated as Jacquard Red Label Silk Colors, but otherwise extremely similar.
An excellent way to dye wool with Remazol type dyes is by immersion, in a cooking pot (one which, like all dyepots, should never again be used for food preparation). You make the dyebath with water, Remazol dye (either Jacquard Red Label Silk Colors or ProChem Liquid Reactive dye, not with Green Label Silk Colors), plus Synthrapol, and an acid (citric acid or vinegar or sodium bisulfate), and boil the wetted wool gently in this dyebath for an hour. Reportedly, the additives in the Green Label Silk Colors should not be boiled in a dyebath, because they produce fumes which can be quite irritating to the user. Steaming is not supposed to create the same level of problems with fumes as boiling in a dyebath, and is more suitable for setting multiple colors of dye on hand-painted roving.
Remazol Dyes are true reactive dyes, not acid dyes, and will form a more permanent bond with wool than acid dyes can do. They can be washed in water at any temperature, many times, without fading.
(Please help support this web site. Thank you.)
Message: My question is...I have been using Jacquard silk colors (green label) to dye my silk fabric and I would like to know if I can use that dye to dye my wool roving as well or do I have to use another type of dye?
—ADVERTISEMENT—
Buy Jacquard Silk Colors from MisterArt.comThe world's largest online discount art & craft supply store!
Jacquard Permanent
Dyeset Concentrate
Jacquard Green Label
Silk Colors
Yes, this should work. Jacquard Green Label Silk Colors are Remazol-type fiber reactive dyes, with acid and some other chemicals added to make it easier to use without steaming. Since wool is a protein fiber like silk, I think that you should be able to use it on wool in just the same way that you do on silk, either with steaming to set the dye, or with the chemical fixative called Jacquard Permanent Dyeset Concentrate. The Permanent Dyeset Concentrate produce slightly less intense colors than those that steaming can produce with the same dye, but is more convenient to use.
For wool, I would prefer to use Jacquard Red Label Silk Colors, or the less expensive (but more concentrated) Liquid Reactive Dyes sold by PRO Chemical & Dye. These all contain the same type of dye. The Jacquard Red Label dyes must usually be special-ordered, from the same places that stock Jacquard Green Label Silk Colors; it is often easier, and always more economical, to order ProChem's version. The advantage of Jacquard Red Label Silk Colors, as compared to the Green Label Silk Colors, is that the Red Label dye is twice as strong as the Green Label dye, and does not contain the chemicals which make Green Label Silk Colors unsuitable for immersion dyeing on the stovetop, due to the fumes that are produced. (Unlike the Red Label Silk Colors, you cannot use Green Label Silk Colors on cotton and other cellulose-containing fibers, because the Green Label dyes contain acid, while cotton requires a high pH to react with these dyes.) Red Label Silk Colors are little besides pure dye, dissolved in water, without the additives of the Green Label dyes; they can be used on any natural fiber. ProChem's Liquid Reactive Remazol dyes are at least four times as concentrated as Jacquard Red Label Silk Colors, but otherwise extremely similar.
An excellent way to dye wool with Remazol type dyes is by immersion, in a cooking pot (one which, like all dyepots, should never again be used for food preparation). You make the dyebath with water, Remazol dye (either Jacquard Red Label Silk Colors or ProChem Liquid Reactive dye, not with Green Label Silk Colors), plus Synthrapol, and an acid (citric acid or vinegar or sodium bisulfate), and boil the wetted wool gently in this dyebath for an hour. Reportedly, the additives in the Green Label Silk Colors should not be boiled in a dyebath, because they produce fumes which can be quite irritating to the user. Steaming is not supposed to create the same level of problems with fumes as boiling in a dyebath, and is more suitable for setting multiple colors of dye on hand-painted roving.
Remazol Dyes are true reactive dyes, not acid dyes, and will form a more permanent bond with wool than acid dyes can do. They can be washed in water at any temperature, many times, without fading.
(Please help support this web site. Thank you.)
Tuesday, December 25, 2007
solving problems with immersion dyeing to a single solid shade
Name: nikki
Message: Hi there, the problem that I have been having when I'm trying to immersion dye for even color. I usually dye one yard in a bucket and I agitate and stir as directed but I cannot find any recipes that call for 1 yard. So usually the end result is certain areas are lighter. What am I doing wrong? Hope to hear from you soon. Peace Nikki
What kind of dye are you using? Since you didn't specify, I will assume that you are using a fiber reactive dye, such as Procion MX dye. Not all of these suggestions will be equally suitable for other types of dye, such as all-purpose dye. There are a number of different details in how you do your dyeing that will help you to get all of your fabric to dye the exact same shade.
How much does your fabric weigh per yard, when dry? The amount of ingredients you need in your dyebath depend on the weight of the fabric, rather than on how many yards long it is. Depending on the thickness of the fabric, it may weigh only a few ounces per yard. Dharma Trading Company has instructions for dyeing eight ounces of fabric. For dyeing four ounces of fabric, you could follow these directions exactly in water volume, but cut the amount of dye and Calsolene oil in half.
I don't usually use Calsolene oil, but if you're having troubles getting your dye to be level (level means dyeing to a single solid shade throughout), it would be a good idea to try it. Calsolene oil is a surfactant which helps the dye to penetrate the fabric more evenly.
How frequently do you stir your fabric? Very frequent stirring, especially continuous stirring for the first ten or fifteen minutes after you add the dye and after you add the soda ash, is very important.
How are you adding your soda ash? You will get the most level results by first stirring the fabric in the dyebath with the dye and for a while, then adding the soda ash only a little at a time. Never pour soda ash directly on your fabric, and do not add it all at once. Some dyers prefer to remove the fabric from the dyebath altogether while adding the soda ash, while others find it sufficient (and less messy) to just hold the fabric to one side of the bucket with a stick while stirring the soda ash into the rest of the dyebath. Always predissolve your soda ash in water before adding it. Add one third of the soda ash, stir the fabric for five minutes, add more soda ash, stir more, and so on.
How big is your bucket? A larger bucket and a larger volume of water helps in getting a more smoothly solid color. A three-gallon bucket is better than a gallon-and-a-half bucket, when level dyeing is important. If adding Calsolene oil is not sufficient to improve your results, using a larger volume of water may do the trick.
Proper prewashing is important in prevent uneven results. Prewash your fabric in very hot water with Synthrapol and some extra soda ash to help remove sizing and other fabric treatments which interfere with level dyeing. If your water supply is not hot enough, consider doing this in a cooking pot on the stove, to make sure that your water is at least 140°F (but do not use soda ash in an aluminum pot). Some treatments, such as permanent press, stain resistance, and starch sizing, are difficult or impossible to remove even with boiling, in which case you are better off with PFD fabric, which has been prepared specifically for dyeing.
(Please help support this web site. Thank you.)
Message: Hi there, the problem that I have been having when I'm trying to immersion dye for even color. I usually dye one yard in a bucket and I agitate and stir as directed but I cannot find any recipes that call for 1 yard. So usually the end result is certain areas are lighter. What am I doing wrong? Hope to hear from you soon. Peace Nikki
What kind of dye are you using? Since you didn't specify, I will assume that you are using a fiber reactive dye, such as Procion MX dye. Not all of these suggestions will be equally suitable for other types of dye, such as all-purpose dye. There are a number of different details in how you do your dyeing that will help you to get all of your fabric to dye the exact same shade.
How much does your fabric weigh per yard, when dry? The amount of ingredients you need in your dyebath depend on the weight of the fabric, rather than on how many yards long it is. Depending on the thickness of the fabric, it may weigh only a few ounces per yard. Dharma Trading Company has instructions for dyeing eight ounces of fabric. For dyeing four ounces of fabric, you could follow these directions exactly in water volume, but cut the amount of dye and Calsolene oil in half.
I don't usually use Calsolene oil, but if you're having troubles getting your dye to be level (level means dyeing to a single solid shade throughout), it would be a good idea to try it. Calsolene oil is a surfactant which helps the dye to penetrate the fabric more evenly.
How frequently do you stir your fabric? Very frequent stirring, especially continuous stirring for the first ten or fifteen minutes after you add the dye and after you add the soda ash, is very important.
How are you adding your soda ash? You will get the most level results by first stirring the fabric in the dyebath with the dye and for a while, then adding the soda ash only a little at a time. Never pour soda ash directly on your fabric, and do not add it all at once. Some dyers prefer to remove the fabric from the dyebath altogether while adding the soda ash, while others find it sufficient (and less messy) to just hold the fabric to one side of the bucket with a stick while stirring the soda ash into the rest of the dyebath. Always predissolve your soda ash in water before adding it. Add one third of the soda ash, stir the fabric for five minutes, add more soda ash, stir more, and so on.
How big is your bucket? A larger bucket and a larger volume of water helps in getting a more smoothly solid color. A three-gallon bucket is better than a gallon-and-a-half bucket, when level dyeing is important. If adding Calsolene oil is not sufficient to improve your results, using a larger volume of water may do the trick.
Proper prewashing is important in prevent uneven results. Prewash your fabric in very hot water with Synthrapol and some extra soda ash to help remove sizing and other fabric treatments which interfere with level dyeing. If your water supply is not hot enough, consider doing this in a cooking pot on the stove, to make sure that your water is at least 140°F (but do not use soda ash in an aluminum pot). Some treatments, such as permanent press, stain resistance, and starch sizing, are difficult or impossible to remove even with boiling, in which case you are better off with PFD fabric, which has been prepared specifically for dyeing.
(Please help support this web site. Thank you.)
Thursday, December 20, 2007
Is there any way to dye with cold water?
Name: Caroline
Message: Is there anyway to dye with cold water? Basically I have some Rit Dye and I want to dye something that is 100% cotton and I am scared it will shrink too much if I dye it in really hot water. I actually want the color to look like a faded black or dark grey and I want to do it in my washing machine. Since I want it to look faded anyway, would doing it in cold water work? Would doubling up on the amount of dye I need help? Thanks
Yes, it is possible to dye cotton with cool water, but NOT with that dye!

Rit dye is a hot water dye, of a type known as all-purpose dye. It works best if you simmer your clothing in it for half an hour or an hour, just below boiling (190°F to 200°F, or 88°C to 93°C). Rit dye is a mixture of hot water leveling acid dyes, which work on wool and nylon, and hot water direct dyes, which work on cotton, but, even when applied correctly, will fade quickly and bleed forever in the laundry.
If you are not going to wash this garment—that is, if it is for a one-time-use costume—then you can get adequate results by staining with Rit dye at room temperature, but the color will wash out when you launder it. I can't recommend this for anything you want to be able to wash and wear repeatedly.
Instead, you need to buy yourself some cold water fiber reactive dye. Then you will be able to dye your cotton clothing at room temperature, assuming your room is 70°F or above. It is very easy to dye in the washing machine with Procion MX dye; see "How can I dye clothing or fabric in the washing machine?".
The best place to buy fiber reactive dye is by mail order, which will give you a wide range of color choices as well as better prices. The most popular type is Procion MX dye. It is very easy to work with, requiring only soda ash (the same chemical as washing soda, a common ingredient in laundry detergent) to set the dye. (Note that soda ash does not work with Rit dye.) For good mail-order sources, see my page of "Sources for Dyeing Supplies Around the World". In the eastern US, where you're located, an excellent source is PRO Chemical & Dye. Dyes purchased by mail-order are far more economical, in the long run, than Rit dye, because one box of Rit dye will dye only a quarter to a half a pound of fabric, whereas a two-ounce jar of Procion MX dye will dye several pounds of fabric.

If you want to buy good dyes at a local store, they will be a little harder to find than Rit dye, and almost as expensive. It's a strange thing that the most unsuitable sort of dye is the easiest to find in local shops in the US. If you have a Joann's fabric store or a very good arts and crafts store nearby, you may be able to find "Dylon Cold" dye, which is mostly Procion MX dye; the accompanying "Dylon Cold Fix" is just soda ash. Tie-dye kits, such as those sold by Jacquard, Tulip, or Dritz, contain high-quality Procion MX type dye, but not the black or gray color that you are looking for. "Dylon Permanent" dye is different from "Dylon Cold" dye; it works best in hot tap water, though it does not require the much higher temperatures that Rit requires. "Dylon Machine Dye" is good for front-loading washing machines, but is not available in the US. Avoid "Dylon Multi Purpose" dye, which is another all-purpose hot water dye.
Whenever you are dyeing anything black, regardless of the type of dye you are using, it is best to use double the amount of dye that you would use for another color, or even four times as much, if you want to get black on your fabric, instead of grey. However, even if you do this with Rit dye, the dye will not be at all permanent in the fabric when used at cool temperatures. Rit dye requires extremely hot water.
There is one possible solution, which
will make Rit dye more permanent on your clothing: you can use a commercial
cationic dye
fixative, such as Retayne, to
set the all-purpose dye. However, this product is almost impossible to find
locally, so you must buy it by mail-order anyway, so you may as well just go
ahead and order yourself some cool water dye, instead. Sometimes you may be able
to find Retayne at your local quilting supply store, or even a Joann's fabric
store. (If you have such a quilting supply store nearby, it is important to
support it by shopping there, as much as possible, instead of buying
mail-order!) It is a good product that is handy to have on hand. However, it
does reduce lightfastness
, so that garments treated with Retayne tend to fade in the sun. Garments
treated with Retayne should be dried indoors, not in direct sunlight. People
ignorant of dyeing will sometimes recommend that you use vinegar or salt to set
Rit dye, but neither vinegar nor salt can do anything to make all-purpose dye
more permanent on cotton.
which
will make Rit dye more permanent on your clothing: you can use a commercial
cationic dye
fixative, such as Retayne, to
set the all-purpose dye. However, this product is almost impossible to find
locally, so you must buy it by mail-order anyway, so you may as well just go
ahead and order yourself some cool water dye, instead. Sometimes you may be able
to find Retayne at your local quilting supply store, or even a Joann's fabric
store. (If you have such a quilting supply store nearby, it is important to
support it by shopping there, as much as possible, instead of buying
mail-order!) It is a good product that is handy to have on hand. However, it
does reduce lightfastness
, so that garments treated with Retayne tend to fade in the sun. Garments
treated with Retayne should be dried indoors, not in direct sunlight. People
ignorant of dyeing will sometimes recommend that you use vinegar or salt to set
Rit dye, but neither vinegar nor salt can do anything to make all-purpose dye
more permanent on cotton.
(Please help support this web site. Thank you.)
Message: Is there anyway to dye with cold water? Basically I have some Rit Dye and I want to dye something that is 100% cotton and I am scared it will shrink too much if I dye it in really hot water. I actually want the color to look like a faded black or dark grey and I want to do it in my washing machine. Since I want it to look faded anyway, would doing it in cold water work? Would doubling up on the amount of dye I need help? Thanks
Yes, it is possible to dye cotton with cool water, but NOT with that dye!

Rit dye is a hot water dye, of a type known as all-purpose dye. It works best if you simmer your clothing in it for half an hour or an hour, just below boiling (190°F to 200°F, or 88°C to 93°C). Rit dye is a mixture of hot water leveling acid dyes, which work on wool and nylon, and hot water direct dyes, which work on cotton, but, even when applied correctly, will fade quickly and bleed forever in the laundry.
If you are not going to wash this garment—that is, if it is for a one-time-use costume—then you can get adequate results by staining with Rit dye at room temperature, but the color will wash out when you launder it. I can't recommend this for anything you want to be able to wash and wear repeatedly.
Instead, you need to buy yourself some cold water fiber reactive dye. Then you will be able to dye your cotton clothing at room temperature, assuming your room is 70°F or above. It is very easy to dye in the washing machine with Procion MX dye; see "How can I dye clothing or fabric in the washing machine?".
The best place to buy fiber reactive dye is by mail order, which will give you a wide range of color choices as well as better prices. The most popular type is Procion MX dye. It is very easy to work with, requiring only soda ash (the same chemical as washing soda, a common ingredient in laundry detergent) to set the dye. (Note that soda ash does not work with Rit dye.) For good mail-order sources, see my page of "Sources for Dyeing Supplies Around the World". In the eastern US, where you're located, an excellent source is PRO Chemical & Dye. Dyes purchased by mail-order are far more economical, in the long run, than Rit dye, because one box of Rit dye will dye only a quarter to a half a pound of fabric, whereas a two-ounce jar of Procion MX dye will dye several pounds of fabric.

If you want to buy good dyes at a local store, they will be a little harder to find than Rit dye, and almost as expensive. It's a strange thing that the most unsuitable sort of dye is the easiest to find in local shops in the US. If you have a Joann's fabric store or a very good arts and crafts store nearby, you may be able to find "Dylon Cold" dye, which is mostly Procion MX dye; the accompanying "Dylon Cold Fix" is just soda ash. Tie-dye kits, such as those sold by Jacquard, Tulip, or Dritz, contain high-quality Procion MX type dye, but not the black or gray color that you are looking for. "Dylon Permanent" dye is different from "Dylon Cold" dye; it works best in hot tap water, though it does not require the much higher temperatures that Rit requires. "Dylon Machine Dye" is good for front-loading washing machines, but is not available in the US. Avoid "Dylon Multi Purpose" dye, which is another all-purpose hot water dye.
Whenever you are dyeing anything black, regardless of the type of dye you are using, it is best to use double the amount of dye that you would use for another color, or even four times as much, if you want to get black on your fabric, instead of grey. However, even if you do this with Rit dye, the dye will not be at all permanent in the fabric when used at cool temperatures. Rit dye requires extremely hot water.
There is one possible solution,
 which
will make Rit dye more permanent on your clothing: you can use a commercial
cationic dye
fixative, such as Retayne, to
set the all-purpose dye. However, this product is almost impossible to find
locally, so you must buy it by mail-order anyway, so you may as well just go
ahead and order yourself some cool water dye, instead. Sometimes you may be able
to find Retayne at your local quilting supply store, or even a Joann's fabric
store. (If you have such a quilting supply store nearby, it is important to
support it by shopping there, as much as possible, instead of buying
mail-order!) It is a good product that is handy to have on hand. However, it
does reduce lightfastness
, so that garments treated with Retayne tend to fade in the sun. Garments
treated with Retayne should be dried indoors, not in direct sunlight. People
ignorant of dyeing will sometimes recommend that you use vinegar or salt to set
Rit dye, but neither vinegar nor salt can do anything to make all-purpose dye
more permanent on cotton.
which
will make Rit dye more permanent on your clothing: you can use a commercial
cationic dye
fixative, such as Retayne, to
set the all-purpose dye. However, this product is almost impossible to find
locally, so you must buy it by mail-order anyway, so you may as well just go
ahead and order yourself some cool water dye, instead. Sometimes you may be able
to find Retayne at your local quilting supply store, or even a Joann's fabric
store. (If you have such a quilting supply store nearby, it is important to
support it by shopping there, as much as possible, instead of buying
mail-order!) It is a good product that is handy to have on hand. However, it
does reduce lightfastness
, so that garments treated with Retayne tend to fade in the sun. Garments
treated with Retayne should be dried indoors, not in direct sunlight. People
ignorant of dyeing will sometimes recommend that you use vinegar or salt to set
Rit dye, but neither vinegar nor salt can do anything to make all-purpose dye
more permanent on cotton.(Please help support this web site. Thank you.)
Wednesday, December 19, 2007
what to use to dye soft white polyurethane foam
Name: lim
Message: Could you guide me as to what to use to dye soft white polyurethane (sponge like) material into a solid color? I saw the disperse dye but there is no polyurethane, tried dylon multi purpose and the color is light and not solid flat. Thank you in advance.
Polyurethane might be better dyed in the manufacturing process, adding the dye to the liquid before it is turned into a foam. If that is not possible, then there are several possible alternatives, but I have not tried any of them on polyurethane foam myself, and can share with you only what I have read. Most questions I'm asked about polyurethane concern stretch fibers, such as Lycra Spandex, which are made from polyurethane. (See How to Dye Spandex.)
Disperse dye, which is used on most synthetic fibers and is the only option for dyeing polyester, can be used to dye polyurethane, but the washfastness is poor, and the heat required may damage the polyurethane. It can be used for pale shades, but I don't recommend that you acquire disperse dye just for this purpose. (See Dyeing Polyester with Disperse Dye.)
The acid dye that makes up a part of Dylon Multi Purpose dye is almost certainly a leveling acid dye, as is found in most all-purpose dyes. What temperature did you apply it at? Perhaps a warmer temperature for a longer period of time, and/or a larger concentration of dye in your dyebath, and/or adjusting the pH with an acid, would help. I would recommend that you try heating it in the dye gradually for an hour or more at 40°C or above (104°F), possibly with the addition of some vinegar (try 100 ml of distilled white vinegar, 5% strength, for every four liters of dyebath). Leveling acid dyes can be used on polyurethane, using a dyebath at temperatures between 40°C and 60°C (104°F and 140°F). The washfastness of leveling acid dyes is not very good, but they tend to produce solid colors more readily, at least on wool; performance on polyurethane might be different. See my page on Leveling Acid Dyes (Kiton type Dyes).
Dylon Multi Purpose dye ought to work, but don't try other sorts of Dylon dye. Dylon Permanent dye, Dylon Hand dye, Dylon Machine dye, and Dylon Cold Water dye are all fiber reactive dyes which work only on natural fibers. Under some circumstances, reactive dyes can be used as acid dyes, but there's no reason to try that unless you are already a user of reactive dyes.
Metal complex dyes (premetallised or premetalized) are more washfast on polyurethane than other acid dyes, but I've read that it is necessary to avoid 1:1 acid dyes because the lower pH required may damage the polyurethane. 1:2 metal complex dyes are good for polyurethane, though the dyes are likely to be less level (less likely to produce a single solid shade). In the US we can buy a 1:2 metal complex dye as ProChem's Washfast Dye in jet black, and in several of the dyes in the Lanaset dye range. (See "About Lanaset Dyes", and "Which Lanaset dye colors are pure, rather than mixtures?". KraftKolour in Australia also sells metal complex dyes for use in hand dyeing; ask them, before buying, which are 1:1 and which 1:2 metal complex dyes. (There are many factories in Asia making these dyes, but most do not sell in quantities small enough to be sutitable for hand dyers.)
Industrially, chrome dyes may be used on polyurethane, with better washfastness, more evenly solid colors, and lower material costs. However, I strongly recommend against the use of chrome dyes, which are used with a separate solution of hexavalent chromium, potassium dichromate, a known human carcinogen and dangerous industrial pollutant. I do not know of a source for chrome dyes for the hand dyers, though dangerous potassium dichromate is often used as a mordant with natural dyes.
If you succeed in dyeing your polyurethane foam, but in spite of your best efforts find that it is not sufficiently washfast afterwards, you should be able to fix the dye with a cationic dye fixative, such as Retayne, or Batik Oetoro's DyeFix. See Commercial Dye Fixatives.
Much of my information on dyeing polyurethane originally came from the book "Blends Dyeing", by John Shore, published by the Society of Dyers and Chemists in the UK.
(Please help support this web site. Thank you.)
Message: Could you guide me as to what to use to dye soft white polyurethane (sponge like) material into a solid color? I saw the disperse dye but there is no polyurethane, tried dylon multi purpose and the color is light and not solid flat. Thank you in advance.
Polyurethane might be better dyed in the manufacturing process, adding the dye to the liquid before it is turned into a foam. If that is not possible, then there are several possible alternatives, but I have not tried any of them on polyurethane foam myself, and can share with you only what I have read. Most questions I'm asked about polyurethane concern stretch fibers, such as Lycra Spandex, which are made from polyurethane. (See How to Dye Spandex.)
Disperse dye, which is used on most synthetic fibers and is the only option for dyeing polyester, can be used to dye polyurethane, but the washfastness is poor, and the heat required may damage the polyurethane. It can be used for pale shades, but I don't recommend that you acquire disperse dye just for this purpose. (See Dyeing Polyester with Disperse Dye.)
The acid dye that makes up a part of Dylon Multi Purpose dye is almost certainly a leveling acid dye, as is found in most all-purpose dyes. What temperature did you apply it at? Perhaps a warmer temperature for a longer period of time, and/or a larger concentration of dye in your dyebath, and/or adjusting the pH with an acid, would help. I would recommend that you try heating it in the dye gradually for an hour or more at 40°C or above (104°F), possibly with the addition of some vinegar (try 100 ml of distilled white vinegar, 5% strength, for every four liters of dyebath). Leveling acid dyes can be used on polyurethane, using a dyebath at temperatures between 40°C and 60°C (104°F and 140°F). The washfastness of leveling acid dyes is not very good, but they tend to produce solid colors more readily, at least on wool; performance on polyurethane might be different. See my page on Leveling Acid Dyes (Kiton type Dyes).
Dylon Multi Purpose dye ought to work, but don't try other sorts of Dylon dye. Dylon Permanent dye, Dylon Hand dye, Dylon Machine dye, and Dylon Cold Water dye are all fiber reactive dyes which work only on natural fibers. Under some circumstances, reactive dyes can be used as acid dyes, but there's no reason to try that unless you are already a user of reactive dyes.
Metal complex dyes (premetallised or premetalized) are more washfast on polyurethane than other acid dyes, but I've read that it is necessary to avoid 1:1 acid dyes because the lower pH required may damage the polyurethane. 1:2 metal complex dyes are good for polyurethane, though the dyes are likely to be less level (less likely to produce a single solid shade). In the US we can buy a 1:2 metal complex dye as ProChem's Washfast Dye in jet black, and in several of the dyes in the Lanaset dye range. (See "About Lanaset Dyes", and "Which Lanaset dye colors are pure, rather than mixtures?". KraftKolour in Australia also sells metal complex dyes for use in hand dyeing; ask them, before buying, which are 1:1 and which 1:2 metal complex dyes. (There are many factories in Asia making these dyes, but most do not sell in quantities small enough to be sutitable for hand dyers.)
Industrially, chrome dyes may be used on polyurethane, with better washfastness, more evenly solid colors, and lower material costs. However, I strongly recommend against the use of chrome dyes, which are used with a separate solution of hexavalent chromium, potassium dichromate, a known human carcinogen and dangerous industrial pollutant. I do not know of a source for chrome dyes for the hand dyers, though dangerous potassium dichromate is often used as a mordant with natural dyes.
If you succeed in dyeing your polyurethane foam, but in spite of your best efforts find that it is not sufficiently washfast afterwards, you should be able to fix the dye with a cationic dye fixative, such as Retayne, or Batik Oetoro's DyeFix. See Commercial Dye Fixatives.
Much of my information on dyeing polyurethane originally came from the book "Blends Dyeing", by John Shore, published by the Society of Dyers and Chemists in the UK.
(Please help support this web site. Thank you.)
Friday, December 07, 2007
problems in dyeing feathers
Name: Jean
Message: I used your instructions to dye feathers, and while the
color was great, the appearance of the feathers was terrible! The individual
fibers (I don't know what to call them of feathers) clumped together and lost
their lovely, feathery appearance. I need to dye some ostrich plumes, and want
to be sure this doesn't happen with them. Thanks.
Which instructions did you follow? I don't believe that I have any instructions on how to dye feathers on my site, except for one Hand Dyeing Q&A posting, "How to Dye Feathers", from February 24, 2005.
That post includes two links, one to Gina Bellous's Feather Tutorial, which uses Jacquard Acid Dyes and vinegar, and PRO Chemical & Dye's instructions for "Staining Silk Flowers and Feathers using PRO WashFast Water-Based Dyes".
Did you have problems with those instructions, or with my instructions on how to dye cotton? Cotton is very different from feathers. Cotton is made of cellulose, while feathers are made of protein. Feathers are dyed with acid dyes, at a low pH, entirely unlike cotton, which is best dyed with fiber reactive dyes and soda ash. The high pH of soda ash is likely to be too rough for feathers.
It certainly is possible to dye feathers without ruining them—this is very popular among fly fishermen—but first you need the right instructions.
Which instructions did you follow? I don't believe that I have any instructions on how to dye feathers on my site, except for one Hand Dyeing Q&A posting, "How to Dye Feathers", from February 24, 2005.
That post includes two links, one to Gina Bellous's Feather Tutorial, which uses Jacquard Acid Dyes and vinegar, and PRO Chemical & Dye's instructions for "Staining Silk Flowers and Feathers using PRO WashFast Water-Based Dyes".
Did you have problems with those instructions, or with my instructions on how to dye cotton? Cotton is very different from feathers. Cotton is made of cellulose, while feathers are made of protein. Feathers are dyed with acid dyes, at a low pH, entirely unlike cotton, which is best dyed with fiber reactive dyes and soda ash. The high pH of soda ash is likely to be too rough for feathers.
It certainly is possible to dye feathers without ruining them—this is very popular among fly fishermen—but first you need the right instructions.
Thursday, December 06, 2007
Can I dye my pants black to hide oily food stains, or will the stains come thru the new dye?
Name: Dan
Message: I have a pair of pants that are weathered black with some small food oil stains that will not wash out. Can I dye them black to hide the stains, or will the stains come thru the new dye? Thanks, Dan
It won't work. The dye will not cover up the stains. It's much like the situation for bleach stains; see "How can I fix the bleach spots on my favorite clothing?", except that the oily stains are darker than the rest of the fabric, rather than lighter, as bleach stains are.
To remove oily stains from your clothing, apply a degreasing cleaner such as PineSol (the original formula which contains pine oil) or LesToil to the stains, making sure it penetrates thoroughly, then launder in the hottest water the clothing can bear. Check the stains after washing, and don't put the clothing through the dryer again until you have managed to remove the stains.
Message: I have a pair of pants that are weathered black with some small food oil stains that will not wash out. Can I dye them black to hide the stains, or will the stains come thru the new dye? Thanks, Dan
It won't work. The dye will not cover up the stains. It's much like the situation for bleach stains; see "How can I fix the bleach spots on my favorite clothing?", except that the oily stains are darker than the rest of the fabric, rather than lighter, as bleach stains are.
To remove oily stains from your clothing, apply a degreasing cleaner such as PineSol (the original formula which contains pine oil) or LesToil to the stains, making sure it penetrates thoroughly, then launder in the hottest water the clothing can bear. Check the stains after washing, and don't put the clothing through the dryer again until you have managed to remove the stains.
Tuesday, December 04, 2007
Would you have any information on potential hue shifts of Lanaset dyes due to fading?
Name: Michael
Message: Would you have any information on potential hue shifts of Lanaset dyes due to fading? I'm interested if Lanaset Blue 5G and Lanaset Green B undergo any hue shifts as they fade.
No, I'm sorry, I don't. It's an interesting question, but not one I have any information on. I have numerical data on lightfastness for many dyes on my page "Lightfastness of Different Types of Dyes", but no notes on changes in hue.
Usually, dyes keep the same hue when they fade, only becoming lighter, as the energy from the light destroys the colorful part of the dye molecule, but this is not necessarily the case for every dye. Occasionally a light-damaged breakdown product of the dye may itself have some color of its own, with a different hue than the original undamaged dye.
Lanaset dyes are more lightfast than many of the alternatives. This is particularly true if you compare them to the French dyes (Pebeo, Sennelier Tinfix, Dupont, etc.), some of which are notably poor in their resistance to light fading.
Linda Knutson, in her book Synthetic Dyes for Natural Fibers, gives a value of 5 to 6, out of 8, for the lightfastness of Lanaset Blue 5G on wool, but does not supply a figure for Lanaset Green B.
You may be able to get information on the lightfastness of your dyes if you contact your supplier. Huntsman, the manufacturer of Lanaset dyes, provides documentation about their dyes online, but only to their customers. Dye manufacturers almost never deal in small quantities such as hand dyers purchase, so the retailer you get your dyes from should request it from them for you, if they do not already have it.
Message: Would you have any information on potential hue shifts of Lanaset dyes due to fading? I'm interested if Lanaset Blue 5G and Lanaset Green B undergo any hue shifts as they fade.
No, I'm sorry, I don't. It's an interesting question, but not one I have any information on. I have numerical data on lightfastness for many dyes on my page "Lightfastness of Different Types of Dyes", but no notes on changes in hue.
Usually, dyes keep the same hue when they fade, only becoming lighter, as the energy from the light destroys the colorful part of the dye molecule, but this is not necessarily the case for every dye. Occasionally a light-damaged breakdown product of the dye may itself have some color of its own, with a different hue than the original undamaged dye.
Lanaset dyes are more lightfast than many of the alternatives. This is particularly true if you compare them to the French dyes (Pebeo, Sennelier Tinfix, Dupont, etc.), some of which are notably poor in their resistance to light fading.
Linda Knutson, in her book Synthetic Dyes for Natural Fibers, gives a value of 5 to 6, out of 8, for the lightfastness of Lanaset Blue 5G on wool, but does not supply a figure for Lanaset Green B.
You may be able to get information on the lightfastness of your dyes if you contact your supplier. Huntsman, the manufacturer of Lanaset dyes, provides documentation about their dyes online, but only to their customers. Dye manufacturers almost never deal in small quantities such as hand dyers purchase, so the retailer you get your dyes from should request it from them for you, if they do not already have it.
Monday, December 03, 2007
Do you have any tips for dyeing fur?

Name:
Georgia
Message: Hi! I recently received a hot pink rabbit fur coat as a present, and it makes me look like a strawberry marshmallow. Do you have any tips for dyeing fur? I've looked all over online....but no one knows anything about it. I'd like to dye it black or a more wearable shade, but I don't have the money to send it to a furrier or anything. Any advice would be GREAT!
This is not going to be an easy beginner's project, I'm afraid. Dyeing fur is made difficult by the water- and heat-sensitivity of the skin to which the fur is attached. If you dye the fur like any other animal fiber, such as wool, by simmering it in a nearly-boiling dyebath, you will end up with hard, shrunken ruined leather behind the fur.

Perhaps the most novice-friendly approach would be to try treating the fur like hair, using a dye commercially sold for use in dyeing hair. This would require many boxes of hair dye, I suppose, and results might be patchy in color. You will probably need to apply a leather conditioner to the skin on the inside of the fur to soften it after the exposure to water.


To use textile dyes, instead, you could try PRO Chemical & Dye's instructions for dyeing leather with Procion MX type dyes. Procion MX dye is an excellent fiber reactive dye for cotton, rayon, and silk, but can also be used on other fibers, given the right recipe. The leather base of the fur seems more vulnerable to damage than the fur itself, so it makes sense to try leather dyeing instructions. One key detail of this recipe is that the maximum temperature recommended is 120°F (49°C), so you won't be getting hard stiff boiled leather. (Do not try to follow this recipe using all-purpose dyes, such as Rit® dye!)
Orco, the Organic Dyestuffs Corporation, sells a complete line of dyes for use in coloring fur. There are dyes which will color fur while leaving the leather backing unchanged, and dyes that will color leather without dyeing fur, as well as dyes which will color both. They have a customer support line, but the question is how small-scale the support they offer is. Most large dye companies are not set up to interact with customers who want only ounces, not many kilograms, of dye.
Be sure to choose a color which is much darker than your pink jacket. Black or dark brown should work well, or even purple. Since dye is transparent, you will not be able to dye your fur to make it a lighter color, only darker.
(Please help support this web site. Thank you.)
Advertisement

Message: Hi! I recently received a hot pink rabbit fur coat as a present, and it makes me look like a strawberry marshmallow. Do you have any tips for dyeing fur? I've looked all over online....but no one knows anything about it. I'd like to dye it black or a more wearable shade, but I don't have the money to send it to a furrier or anything. Any advice would be GREAT!
This is not going to be an easy beginner's project, I'm afraid. Dyeing fur is made difficult by the water- and heat-sensitivity of the skin to which the fur is attached. If you dye the fur like any other animal fiber, such as wool, by simmering it in a nearly-boiling dyebath, you will end up with hard, shrunken ruined leather behind the fur.

Perhaps the most novice-friendly approach would be to try treating the fur like hair, using a dye commercially sold for use in dyeing hair. This would require many boxes of hair dye, I suppose, and results might be patchy in color. You will probably need to apply a leather conditioner to the skin on the inside of the fur to soften it after the exposure to water.

To use textile dyes, instead, you could try PRO Chemical & Dye's instructions for dyeing leather with Procion MX type dyes. Procion MX dye is an excellent fiber reactive dye for cotton, rayon, and silk, but can also be used on other fibers, given the right recipe. The leather base of the fur seems more vulnerable to damage than the fur itself, so it makes sense to try leather dyeing instructions. One key detail of this recipe is that the maximum temperature recommended is 120°F (49°C), so you won't be getting hard stiff boiled leather. (Do not try to follow this recipe using all-purpose dyes, such as Rit® dye!)
Orco, the Organic Dyestuffs Corporation, sells a complete line of dyes for use in coloring fur. There are dyes which will color fur while leaving the leather backing unchanged, and dyes that will color leather without dyeing fur, as well as dyes which will color both. They have a customer support line, but the question is how small-scale the support they offer is. Most large dye companies are not set up to interact with customers who want only ounces, not many kilograms, of dye.
Be sure to choose a color which is much darker than your pink jacket. Black or dark brown should work well, or even purple. Since dye is transparent, you will not be able to dye your fur to make it a lighter color, only darker.
(Please help support this web site. Thank you.)
Advertisement
Sunday, December 02, 2007
Can I substitute guar gum for alginate in screen printing with Procion MX type dyes?
Name: Mimi
Message: I have read your site a hundred times over and keep coming back here looking for the answer. I would like to use my fiber reactive/ procion mx dyes that I got a dharma trading company for screen printing but I can't seem to get a straight answer from anywhere. I saw that you mentioned guar gum as a thickener, well I have that and wanted to know if I could use it instead of the sodium alginate or is it absolutely mandatory to use the sodium alginate? If I was to use the guar gum would I follow the same recipe and steps that I have seen for the regular sodium alginate? Thanks for your time and consideration.
It's likely that you will have more difficulty in getting deep colors with guar gum than with alginate, when printing with MX dyes.
It was not in the FAQ, but here's a relevant post from this Hand Dyeing Q&A blog on April 29, 2005: "Is it possible to thicken dye?":
Also see my page entitled "Sodium alginate, Superclear, and other dye thickeners ", from the auxilliary chemicals section of my FAQ. (I've just added a couple of clarifying sentences to the section on guar gum; it was only a single sentence before, which did not mention fiber reactive dyes.)
If you are just getting started with using dye for screen printing, I would recommend that you use a published recipe and follow it carefully. After you have mastered it, then try substitutions and variations on the recipe, such as by substituting guar gum for alginate. If you are already experienced with screen printing, you will find it pretty easy to adapt to using dye instead of ink.
You will need to use soda ash at some point in the process, unless you are going to let your dye dry and then paint on sodium silicate (AfterFix). You can soak your cotton garments in soda ash solution and let them dry, then screen on your thickened dyes. Use a recipe that contains urea so that the dyes will remain moist on the fabric long enough to react with it, and be sure that you allow enough time in a warm enough room (at least 70°F overnight, or warmer temperatures for shorter periods of time).

There is a book on painting and printing with Procion MX dyes that I recommend, by Ann Johnston, Color by Design: Paint and Print with Dye. You might want to take a look at it. Also, look at this "silkscreening with dye" discussion in the Dye Forum on my site. Jane Dunnewold has a book and a DVD that are both entitled Improvisational Screen Printing; see the store section of her website at Art Cloth Studios. Both Ann Johnston and Jane Dunnewold's instructions are highly recommended by both beginning and experienced dyers.
(Please help support this web site. Thank you.)
Message: I have read your site a hundred times over and keep coming back here looking for the answer. I would like to use my fiber reactive/ procion mx dyes that I got a dharma trading company for screen printing but I can't seem to get a straight answer from anywhere. I saw that you mentioned guar gum as a thickener, well I have that and wanted to know if I could use it instead of the sodium alginate or is it absolutely mandatory to use the sodium alginate? If I was to use the guar gum would I follow the same recipe and steps that I have seen for the regular sodium alginate? Thanks for your time and consideration.
It's likely that you will have more difficulty in getting deep colors with guar gum than with alginate, when printing with MX dyes.
It was not in the FAQ, but here's a relevant post from this Hand Dyeing Q&A blog on April 29, 2005: "Is it possible to thicken dye?":
"Guar gum is not ideal, because it reacts with fiber reactive dyes, reducing the amount of dye that actually reaches your fabric. Most people who thicken their Procion MX dyes use either sodium alginate or Superclear. There are two types of alginate, F and SH. Alginate F is used for silk, and alginate SH is used for cotton....You can use guar gum for thickening acid dyes, when dyeing animal fibers (wool or silk) or nylon. Acid dyes cannot be used on cotton."
Also see my page entitled "Sodium alginate, Superclear, and other dye thickeners ", from the auxilliary chemicals section of my FAQ. (I've just added a couple of clarifying sentences to the section on guar gum; it was only a single sentence before, which did not mention fiber reactive dyes.)

If you are just getting started with using dye for screen printing, I would recommend that you use a published recipe and follow it carefully. After you have mastered it, then try substitutions and variations on the recipe, such as by substituting guar gum for alginate. If you are already experienced with screen printing, you will find it pretty easy to adapt to using dye instead of ink.
You will need to use soda ash at some point in the process, unless you are going to let your dye dry and then paint on sodium silicate (AfterFix). You can soak your cotton garments in soda ash solution and let them dry, then screen on your thickened dyes. Use a recipe that contains urea so that the dyes will remain moist on the fabric long enough to react with it, and be sure that you allow enough time in a warm enough room (at least 70°F overnight, or warmer temperatures for shorter periods of time).

There is a book on painting and printing with Procion MX dyes that I recommend, by Ann Johnston, Color by Design: Paint and Print with Dye. You might want to take a look at it. Also, look at this "silkscreening with dye" discussion in the Dye Forum on my site. Jane Dunnewold has a book and a DVD that are both entitled Improvisational Screen Printing; see the store section of her website at Art Cloth Studios. Both Ann Johnston and Jane Dunnewold's instructions are highly recommended by both beginning and experienced dyers.
(Please help support this web site. Thank you.)
Saturday, December 01, 2007
thermochromic pigment changes color when warm, and changes back again when cool
Name: yuvaraj
Message: There is a type of dye that, after dyeing, changes its colour: in heat, pink, and in cold temperatures, violet. I just saw a small swatch. My question is what class of dyes is it & how to dye.
What you're talking about is thermochromic pigment (sometimes referred to as thermochromatic). In the case that you saw, a thermochromic ink was used that is blue when cool and colorless when hot, combined with an ordinary pink dye on the same fabric.
I do not believe that this is a true textile dye; that is, the molecule that gives the color does not itself attach to the fiber directly. Instead, it is a pigment, which requires a binder, such as some sort of acrylic, to attach it to the fiber. This means that, in the context of textiles, it is actually a fabric paint, not a dye. The particle sizes of thermochromic pigments are larger than those in other pigment dyes, so it will be necessary to pay attention to the manufacturer's suggestions when applying it.
My experience with a couple of garments that had been commercially colored with Hypercolor thermochromic pigment was one of poor washfastness. The garments did their color change well only for a short time. After a few weeks (of weekly launderings), they faded out and no longer made the color change. However, the thermochromic effect was fun enough to be worth trying even for a short-term effect. I've seen many more non-textile objects with thermochromic ink, such as coffee mugs that change their decoration when filled with a hot liquid. Thermochromic clothing is now available in the US from Dyenamic Infusion, which claims improved washfastness for their line of color-changing shirts.

So far, I have seen only one source for small quantities of do-it-yourself thermochromic fabric paint, in the UK, at Ridgewell Press Smart Textiles. [Update: link to internet archive page.] They do ship internationally, but, if you are not in Europe, you must contact them to find out what the postage will cost. Here are their instructions:
On the same page they sell UV-reactive pigments which are colorless indoors, but turn into bright colors in the sunlight. This is a different phenomenon than that of fluorescence, in which a dye or pigment absorbs one wavelength of light (usually invisible ultraviolet) and then emits that same energy as another wavelength. Instead, the UV-reactive pigments actually change their structure to a colored form, under the influence of ultraviolet light, after which the additional visible light which reaches the pigment is reflected so as to produce a color.
There are other sources for thermochromic inks, but these will not be washproof when used on fabric, unless the manufacturer specifically claims that they will. Look for thermochromic plastisol inks that are intended for use in printing t-shirts. These will feel more like plastic on the fabric, like many commercially screen-printed t-shirts, instead of like dye. I wonder whether they might be more resistant to damage in the laundry than the old Hypercolor pigment-dyed shirts. Always wash fabric-painted garments with care, turning them inside out before laundering, and placing them in a net lingerie bag for additional protection against wear. Thermochromic inks should probably, in addition, be washed only in cool water, and drip-dried, not machine-dried, as this might help to extend their working lives.
How does it work? There are two different types of thermochromic inks, one based on liquid crystals and the other based on leucodyes. Liquid crystals are impractical to work with in textiles, so we can ignore them. Leucodyes are chemically part of the vat dye family; the most familiar vat dye is indigo dye. Thermochromic leucodye mixtures are quite different from indigo, however. There are many different dyes that change color when the pH changes. The clever thing about thermochromic inks is that the mixture the dye is placed in causes the pH around the dye ingredient to change when the temperature changes. The leucodye mixtures are microencapsulated in a protective coating: a leucodye that changes color when the pH changes, along with a fatty acid/solvent that will reversibly change state upon exposure to heat, and a weak acid that will change the pH of the solution when the fatty acid is melted. The relatively large pigment particles that result are then mixed with compatible binders and other materials to create the thermochromic ink. Thermochromic inks are very expensive in comparison to ordinary inks, but have many interesting and useful applications. For more information see a 2003 article written by Timothy J. Homola, "Color-Changing Inks: Brighten your bottom line". [Updated link.] Also see Wikipedia's surprisingly well-written article on Thermochromism, as well as one on the Hypercolor line of clothing.
Chapter 10 of John Crano and Robert Guglielmetti's
book, Organic Photochromic and Thermochromic Compounds: Volume 2:
Physicochemical Studies, Biological Applications, and Thermochromism,
goes into more detail about the chemistry of thermochromic compounds, including
"spiroheterocyclic compounds, Schiff bases and related nitrogen-containing
molecules, bianthrones and other overcrowded ethenes, and miscellaneous
compounds." It's a very expensive book, but perhaps you could find it at the
library of a university or other institution.
John Crano and Robert Guglielmetti's
book, Organic Photochromic and Thermochromic Compounds: Volume 2:
Physicochemical Studies, Biological Applications, and Thermochromism,
goes into more detail about the chemistry of thermochromic compounds, including
"spiroheterocyclic compounds, Schiff bases and related nitrogen-containing
molecules, bianthrones and other overcrowded ethenes, and miscellaneous
compounds." It's a very expensive book, but perhaps you could find it at the
library of a university or other institution.
(Please help support this web site. Thank you.)
Message: There is a type of dye that, after dyeing, changes its colour: in heat, pink, and in cold temperatures, violet. I just saw a small swatch. My question is what class of dyes is it & how to dye.
What you're talking about is thermochromic pigment (sometimes referred to as thermochromatic). In the case that you saw, a thermochromic ink was used that is blue when cool and colorless when hot, combined with an ordinary pink dye on the same fabric.
I do not believe that this is a true textile dye; that is, the molecule that gives the color does not itself attach to the fiber directly. Instead, it is a pigment, which requires a binder, such as some sort of acrylic, to attach it to the fiber. This means that, in the context of textiles, it is actually a fabric paint, not a dye. The particle sizes of thermochromic pigments are larger than those in other pigment dyes, so it will be necessary to pay attention to the manufacturer's suggestions when applying it.
My experience with a couple of garments that had been commercially colored with Hypercolor thermochromic pigment was one of poor washfastness. The garments did their color change well only for a short time. After a few weeks (of weekly launderings), they faded out and no longer made the color change. However, the thermochromic effect was fun enough to be worth trying even for a short-term effect. I've seen many more non-textile objects with thermochromic ink, such as coffee mugs that change their decoration when filled with a hot liquid. Thermochromic clothing is now available in the US from Dyenamic Infusion, which claims improved washfastness for their line of color-changing shirts.

So far, I have seen only one source for small quantities of do-it-yourself thermochromic fabric paint, in the UK, at Ridgewell Press Smart Textiles. [Update: link to internet archive page.] They do ship internationally, but, if you are not in Europe, you must contact them to find out what the postage will cost. Here are their instructions:
"Using thermochromic fabric paint
The thermochromic paint is already mixed with an acrylic binder so it can be painted or screen printed directly onto the fabric. Choose light coloured fabrics for this work. If you use dark fabrics you will not be able to see the colour change.
"Dry the fabric, heat seal it with an iron or heat press to fix the dye and the fabric is ready to use. Put a piece of parchment or greaseproof paper on top of the fabric and iron with a hot iron for a short time.
"Thermochromic dyes are most effective when used over another lighter colour, so you could use the orange on top of yellow, or the blue on top of light green."
On the same page they sell UV-reactive pigments which are colorless indoors, but turn into bright colors in the sunlight. This is a different phenomenon than that of fluorescence, in which a dye or pigment absorbs one wavelength of light (usually invisible ultraviolet) and then emits that same energy as another wavelength. Instead, the UV-reactive pigments actually change their structure to a colored form, under the influence of ultraviolet light, after which the additional visible light which reaches the pigment is reflected so as to produce a color.
There are other sources for thermochromic inks, but these will not be washproof when used on fabric, unless the manufacturer specifically claims that they will. Look for thermochromic plastisol inks that are intended for use in printing t-shirts. These will feel more like plastic on the fabric, like many commercially screen-printed t-shirts, instead of like dye. I wonder whether they might be more resistant to damage in the laundry than the old Hypercolor pigment-dyed shirts. Always wash fabric-painted garments with care, turning them inside out before laundering, and placing them in a net lingerie bag for additional protection against wear. Thermochromic inks should probably, in addition, be washed only in cool water, and drip-dried, not machine-dried, as this might help to extend their working lives.
How does it work? There are two different types of thermochromic inks, one based on liquid crystals and the other based on leucodyes. Liquid crystals are impractical to work with in textiles, so we can ignore them. Leucodyes are chemically part of the vat dye family; the most familiar vat dye is indigo dye. Thermochromic leucodye mixtures are quite different from indigo, however. There are many different dyes that change color when the pH changes. The clever thing about thermochromic inks is that the mixture the dye is placed in causes the pH around the dye ingredient to change when the temperature changes. The leucodye mixtures are microencapsulated in a protective coating: a leucodye that changes color when the pH changes, along with a fatty acid/solvent that will reversibly change state upon exposure to heat, and a weak acid that will change the pH of the solution when the fatty acid is melted. The relatively large pigment particles that result are then mixed with compatible binders and other materials to create the thermochromic ink. Thermochromic inks are very expensive in comparison to ordinary inks, but have many interesting and useful applications. For more information see a 2003 article written by Timothy J. Homola, "Color-Changing Inks: Brighten your bottom line". [Updated link.] Also see Wikipedia's surprisingly well-written article on Thermochromism, as well as one on the Hypercolor line of clothing.
Chapter 10 of
 John Crano and Robert Guglielmetti's
book, Organic Photochromic and Thermochromic Compounds: Volume 2:
Physicochemical Studies, Biological Applications, and Thermochromism,
goes into more detail about the chemistry of thermochromic compounds, including
"spiroheterocyclic compounds, Schiff bases and related nitrogen-containing
molecules, bianthrones and other overcrowded ethenes, and miscellaneous
compounds." It's a very expensive book, but perhaps you could find it at the
library of a university or other institution.
John Crano and Robert Guglielmetti's
book, Organic Photochromic and Thermochromic Compounds: Volume 2:
Physicochemical Studies, Biological Applications, and Thermochromism,
goes into more detail about the chemistry of thermochromic compounds, including
"spiroheterocyclic compounds, Schiff bases and related nitrogen-containing
molecules, bianthrones and other overcrowded ethenes, and miscellaneous
compounds." It's a very expensive book, but perhaps you could find it at the
library of a university or other institution. (Please help support this web site. Thank you.)


