« 2008 June | Main | 2008 April »
Wednesday, May 07, 2008
How can I make dyes out of just the general reagents available in an ordinary chemistry lab?
Tuesday, May 06, 2008
What is the dye used for clothing typically made from?
Monday, May 05, 2008
Can one set the colors for tie dying with vinegar when the project is complete rather than buying the sodium carbonate and soaking the fabric before dying?
Sunday, May 04, 2008
Procion MX dye in Italy?
Saturday, May 03, 2008
Can I redye a canvas boat cover that faded in the sun?
Friday, May 02, 2008
Can I overdye green coveralls to make them blue?
Thursday, May 01, 2008
How do I clean a batik before reframing it?
Saturday, May 31, 2008
Is there a specific name for Shibori dyeing using pebbles and beans?
Friday, May 30, 2008
Can you dye clothes with a Sharpie?
Thursday, May 29, 2008
What polyester should I buy for transfer printing?
Wednesday, May 28, 2008
What can you do if you forgot to use soda ash and the dye is rinsing out?
Tuesday, May 27, 2008
tie-dyeing on already black t-shirts
Monday, May 26, 2008
steaming dry silk with food dyes
Sunday, May 25, 2008
Is it possible to tie-dye black t-shirts with white?
Thursday, May 22, 2008
I really need to know how to tie the mirror spiral shirt
Wednesday, May 21, 2008
Prior to overdyeing, do I resoak these fabric pieces in soda ash once again?
Tuesday, May 20, 2008
How long can you store dye premix solution that hasn't been mixed with any dye?
Monday, May 19, 2008
How long can you keep procion MX dyes, after you mix them with water and urea?
Sunday, May 18, 2008
neutralizing sodium hypochlorite with sodium metabifulfite
Saturday, May 17, 2008
Although the linen has dyed successfully the stitching has not taken the colour and they don't look any good!
Friday, May 16, 2008
Dyeing variegated wool with Lanaset dyes
Wednesday, May 14, 2008
I want to dye my swimsuit. It's 80% polyester and 20% spandex. Would disperse dye work?
Tuesday, May 13, 2008
I have bought a viscose "denim coloured" two piece, and I was wondering if there is any treatment that would hold the colour
Can the shirts stay over the weekend in plastic to remain wet after dyeing (tie dyeing) before rinsing?
Monday, May 12, 2008
how to dye a black t-shirt to give it a more vintage distressed look
Sunday, May 11, 2008
Do you take special orders? I'd like a shirt made, but not sure about finding someone to do it.
Saturday, May 10, 2008
I have red cotton slipcovers that have faded from sun and washing
Friday, May 09, 2008
Can I dye a table cloth (100% polyester) that is red to a Navy Blue?
Thursday, May 08, 2008
How can I get a solid even color when dyeing nylon gloves?
To salvage the unevenly dyed gloves, try soaking in boiling water. Some acid dyes will come out when treated this way. If this does not work, and the gloves are ruined anyway by the uneven color, you may try a sulfur-based dye remover, such as Rit Color Remover, or Dylon Run Away. Do not use chlorine (hypochlorite) bleach, because it destroys nylon.
Periwinkle 615 is a premixed dye, containing two or more other dye colors in it, so I don't know which dyes are in it. Hot Fuchsia 620 is acid red 52, a fluorescent dye also known as Rhodamine B. Rhodamine B is also among the dyes ProChem in the US sells in their Washfast Acid line of dyes. In their instructions they say to wet out the fiber by measuring one-half teaspoon (2.5 ml) Synthrapol in 2.5 gallons (10 liters) of warm 110°F (44°C) water, for each pound (454 gm) of fiber, and soak for at least 30 minutes. Take a look at their instructions. They say to use eleven tablespoons of white vinegar, which is 165 ml, for every 10 liters of water, and they say to include a teaspoon of Synthrapol detergent in the dyebath. The recipe is mostly pretty similar to the Jacquard recipe. If you do not have Synthrapol, use liquid hand dishwashing detergent. This should help with dye penetration and evenness.
(Please help support this web site. Thank you.)
Is there a specific name for Shibori dyeing using pebbles and beans?
Name:
melisa
Message: Is there a specific name for Shibori dyeing using pebbles and beans (or similar forms)?
If there is, I have not seen it. I have seen this method referred to in various places, such as the book Memory on Cloth by Yoshiko Wada, and pictured in Kate Wells's book, Fabric Dyeing & Printing, but it's described there only as bound-resist shibori. The name for bound resists in general is kanoko shibori.
I had imagined that mame-shibori might be the name for this resist method, since "mame" is Japanese for "bean", but have tentatively concluded that mame-shibori probably does not usually include the use of an actual bean or other object, but instead only a repetitive and highly laborious series of ties to make small dots. (The ties are cleverly made to be released easily afterwards.) The result of binding a soybean or marble and then immersion-dyeing is a resisted circle, not a dot.
(Please help support this web site. Thank you.)
—ADVERTISEMENT—
Message: Is there a specific name for Shibori dyeing using pebbles and beans (or similar forms)?
—ADVERTISEMENT—
Shibori DVDIf there is, I have not seen it. I have seen this method referred to in various places, such as the book Memory on Cloth by Yoshiko Wada, and pictured in Kate Wells's book, Fabric Dyeing & Printing, but it's described there only as bound-resist shibori. The name for bound resists in general is kanoko shibori.
I had imagined that mame-shibori might be the name for this resist method, since "mame" is Japanese for "bean", but have tentatively concluded that mame-shibori probably does not usually include the use of an actual bean or other object, but instead only a repetitive and highly laborious series of ties to make small dots. (The ties are cleverly made to be released easily afterwards.) The result of binding a soybean or marble and then immersion-dyeing is a resisted circle, not a dot.
(Please help support this web site. Thank you.)
Friday, May 30, 2008
Can you dye clothes with a Sharpie?
Can you dye clothes with a
Sharpie?
A Sharpie marker can be used to temporarily color small sections of clothing. It contains pigments, rather than dyes, and will gradually fade in the laundry, though it will never wash out completely.
Markers labeled specifically for use on fabric will last much longer. Try a fabric marker, such as the ones made by Fabricmate or Marvy.
The best and easiest way to dye cotton clothing is with fiber reactive dye.
(Please help support this web site. Thank you.)
—ADVERTISEMENTS—
Fabric MarkersA Sharpie marker can be used to temporarily color small sections of clothing. It contains pigments, rather than dyes, and will gradually fade in the laundry, though it will never wash out completely.
Markers labeled specifically for use on fabric will last much longer. Try a fabric marker, such as the ones made by Fabricmate or Marvy.
The best and easiest way to dye cotton clothing is with fiber reactive dye.
(Please help support this web site. Thank you.)
Thursday, May 29, 2008
What polyester should I buy for transfer printing?
Name:
Elsbeth
Message: After seeing Judy Coats Perez' "Tea & Entomology" in Quilting Arts #32 I would like to try transfer printing on polyester fabric. Buying transfer paint online was easy, however I cannot find a source for medium weight polyester (the one Judy recommends). There also seem to be many kinds of polyester. Could you help me along?
Any polyester will work for transfer dyeing with disperse dyes, but the weave will make a difference in how well your design shows up. Choose a plain white polyester fabric that you like the feel of. A very smooth satin weave will show finer details than a rougher weave will. Polyester microsuede or fleece will show much less detail, but might be very worthwhile all the same in some projects.
I recommend that you buy a small quantity of each of several white polyester fabrics from your local sewing store, anything that appeals to you. Prewash the fabrics as you would normally wash laundry, following the care instructions found on the end of the bolt (that the fabric is wrapped around before you buy it). After drying the polyester samples, try a quick test of a method similar to that you want to use. There is no better way to get a feel for what it is that you really want.
Or, you can just buy one medium- to heavy-weight polyester satin from the sewing store, not too sheer and not too stiff. A heavier fabric will be able to hold more dye than a lighter fabric. Thin sheer polyesters, such as chiffon, will not produce colors that are nearly as dark or as intense, but they can be quite satisfactory for certain effects, especially for layering over a thicker material.
Other synthetic fibers will also work for transfer dyes. Do not use rayon, which is a cellulose fiber that does not contain a synthetic polymer (rayon should be dyed just like cotton, instead). You can use nylon, acetate, or acrylic. Some fabrics, particularly modacrylic and polypropylene, will melt under the high heat of the transfer press (or home iron) that is used to transfer the disperse dye. Be sure to do a small-scale test before embarking on your main project. It takes very little time to do a small test, and it can save you a lot of disappointment.
If you do not have a local sewing store and must purchase by mail-order, one good choice would be the crepe back satin polyester sold by Joann.com
polyester sold by Joann.com .
.
(Please help support this web site. Thank you.)
Message: After seeing Judy Coats Perez' "Tea & Entomology" in Quilting Arts #32 I would like to try transfer printing on polyester fabric. Buying transfer paint online was easy, however I cannot find a source for medium weight polyester (the one Judy recommends). There also seem to be many kinds of polyester. Could you help me along?
Any polyester will work for transfer dyeing with disperse dyes, but the weave will make a difference in how well your design shows up. Choose a plain white polyester fabric that you like the feel of. A very smooth satin weave will show finer details than a rougher weave will. Polyester microsuede or fleece will show much less detail, but might be very worthwhile all the same in some projects.
I recommend that you buy a small quantity of each of several white polyester fabrics from your local sewing store, anything that appeals to you. Prewash the fabrics as you would normally wash laundry, following the care instructions found on the end of the bolt (that the fabric is wrapped around before you buy it). After drying the polyester samples, try a quick test of a method similar to that you want to use. There is no better way to get a feel for what it is that you really want.
Or, you can just buy one medium- to heavy-weight polyester satin from the sewing store, not too sheer and not too stiff. A heavier fabric will be able to hold more dye than a lighter fabric. Thin sheer polyesters, such as chiffon, will not produce colors that are nearly as dark or as intense, but they can be quite satisfactory for certain effects, especially for layering over a thicker material.
Other synthetic fibers will also work for transfer dyes. Do not use rayon, which is a cellulose fiber that does not contain a synthetic polymer (rayon should be dyed just like cotton, instead). You can use nylon, acetate, or acrylic. Some fabrics, particularly modacrylic and polypropylene, will melt under the high heat of the transfer press (or home iron) that is used to transfer the disperse dye. Be sure to do a small-scale test before embarking on your main project. It takes very little time to do a small test, and it can save you a lot of disappointment.
If you do not have a local sewing store and must purchase by mail-order, one good choice would be the crepe back satin
(Please help support this web site. Thank you.)
Wednesday, May 28, 2008
What can you do if you forgot to use soda ash and the dye is rinsing out?
Name: Shirley
Message: Hey Paula, I hope you can help me!?
I have just dyed some cotton fabric with a fibre reactive dye colour turquoise, thought I had followed all the instructions using soda ash as the fixer but when I got the cotton wet again all the dye started coming out so does not appear to have fixed properly. I couldn't find anything on your site about re fixing after you have dyed it so not sure if it is possible to refix the dried dyed fabric again with more sofa ash? If it is what should I do? Many thanks, Shirley
It's possible that all is perfectly well in your case, and you are just seeing the excess unattached dye washing out of the fabric. Fiber reactive dyes on cotton are very different from acid dyes on wool; there is always a lot of unattached extra dye, which must be removed by washing. However, if almost all of the dye is washing out, you have a real problem. Maybe you left out the soda ash step.
A friend of mine did that once, dyeing cotton with Procion MX dyes without any soda ash at all. What she'd thought was soda ash was actually a urea solution (perhaps the person working with her may have gotten the two mixed up). So, what she did was she soaked the stuff in soda ash, just as you might do a presoak before tie-dyeing. You can use anywhere from one-third to one cup of soda ash per gallon of water.
You will need to take care of this as soon as possible. You want to do it before any more of the dye on the fabric hydrolyzes ("spoils") so that it cannot react with the cotton.
When you put your dyed items into a bucket of soda ash, the dye that has dried on the fabric will run, and may transfer to other parts of the fabric, a real problem if you've been tie-dyeing or dye-painting. To reduce the amount of dye that dissolves in the soda ash solution, add a lot of salt, as much salt as you can possibly dissolve. This is called a saturated salt solution. If a little salt remains undissolved at the bottom, that's fine. That's about 2.4 pounds of salt per gallon [4 liters]; use a little extra to be on the safe side.
You can use this soda ash/saturated salt solution to soak your fabric in, or you can spray it on until the fabric is wet. Then the key is to allow time all over again for the dye reaction to progress, say overnight at 70° or higher. Maintain moisture during this time, either by using urea in the soda ash mixture (one tablespoon per cup, or one cup per gallon), or wrap in plastic, so that the fabric does not dry out.
An alternative method, as described in the "Is there any alternative for soda ash?" section of my page on "What is soda ash, and what's it for in dyeing?" is to buy some sodium silicate solution, which is sold by Dharma Trading Company as AfterFix and as PRO Fix LHF and PRO QuickFix by PRO Chemical & Dye, as well as by other dye suppliers under other names. Sodium silicate works well, with its high pH, to activate the cotton to react with the dye. However, I don't think you should wait to order this stuff. Soda ash with salt should be fine. Don't bother to use salt if your fabric is a single solid color, as the salt is only intended to reduce dye transfer.
It is possible that your turquoise has begun to go bad in the jar. When fiber reactive dyes are in storage, they can, slowly, begin to react with tiny amounts of residual moisture. Once the dye has hydrolyzed, it will show the same behavior of refusing to fix to the cotton, even if soda ash is present and the temperature is adequate. Fiber reactive dyes last for a year or two after purchase, if stored in tightly closed jars, but they will go bad in a single day if stored in a hot enough place, such as a hot car in the sun. Please do a small test with your turquoise before you use it again. If this after-fixing works well, then you know it's okay. I must also mention that the Procion MX type turquoise is more temperature-dependent than the other colors in that series of dyes, so poor turquoise reaction often indicates inadequate warmth in the dye studio. Normally, any temperature over 70°F (21°C) is warm enough if you allow your dye reactions to progress long enough.
Even after Procion MX and other fiber reactive dyes have completely hydrolyzed and are useless for dyeing cotton, they can still be used as acid dyes, on silk, wool, or nylon, if you substitute an acid such as vinegar or citric acid for the soda ash, and apply extra heat. See "Using Fiber reactive dyes on protein fibers". Acid dyes will not work on cotton, however.
(Please help support this web site. Thank you.)
Message: Hey Paula, I hope you can help me!?
I have just dyed some cotton fabric with a fibre reactive dye colour turquoise, thought I had followed all the instructions using soda ash as the fixer but when I got the cotton wet again all the dye started coming out so does not appear to have fixed properly. I couldn't find anything on your site about re fixing after you have dyed it so not sure if it is possible to refix the dried dyed fabric again with more sofa ash? If it is what should I do? Many thanks, Shirley
It's possible that all is perfectly well in your case, and you are just seeing the excess unattached dye washing out of the fabric. Fiber reactive dyes on cotton are very different from acid dyes on wool; there is always a lot of unattached extra dye, which must be removed by washing. However, if almost all of the dye is washing out, you have a real problem. Maybe you left out the soda ash step.
A friend of mine did that once, dyeing cotton with Procion MX dyes without any soda ash at all. What she'd thought was soda ash was actually a urea solution (perhaps the person working with her may have gotten the two mixed up). So, what she did was she soaked the stuff in soda ash, just as you might do a presoak before tie-dyeing. You can use anywhere from one-third to one cup of soda ash per gallon of water.
You will need to take care of this as soon as possible. You want to do it before any more of the dye on the fabric hydrolyzes ("spoils") so that it cannot react with the cotton.
When you put your dyed items into a bucket of soda ash, the dye that has dried on the fabric will run, and may transfer to other parts of the fabric, a real problem if you've been tie-dyeing or dye-painting. To reduce the amount of dye that dissolves in the soda ash solution, add a lot of salt, as much salt as you can possibly dissolve. This is called a saturated salt solution. If a little salt remains undissolved at the bottom, that's fine. That's about 2.4 pounds of salt per gallon [4 liters]; use a little extra to be on the safe side.
You can use this soda ash/saturated salt solution to soak your fabric in, or you can spray it on until the fabric is wet. Then the key is to allow time all over again for the dye reaction to progress, say overnight at 70° or higher. Maintain moisture during this time, either by using urea in the soda ash mixture (one tablespoon per cup, or one cup per gallon), or wrap in plastic, so that the fabric does not dry out.
An alternative method, as described in the "Is there any alternative for soda ash?" section of my page on "What is soda ash, and what's it for in dyeing?" is to buy some sodium silicate solution, which is sold by Dharma Trading Company as AfterFix and as PRO Fix LHF and PRO QuickFix by PRO Chemical & Dye, as well as by other dye suppliers under other names. Sodium silicate works well, with its high pH, to activate the cotton to react with the dye. However, I don't think you should wait to order this stuff. Soda ash with salt should be fine. Don't bother to use salt if your fabric is a single solid color, as the salt is only intended to reduce dye transfer.
It is possible that your turquoise has begun to go bad in the jar. When fiber reactive dyes are in storage, they can, slowly, begin to react with tiny amounts of residual moisture. Once the dye has hydrolyzed, it will show the same behavior of refusing to fix to the cotton, even if soda ash is present and the temperature is adequate. Fiber reactive dyes last for a year or two after purchase, if stored in tightly closed jars, but they will go bad in a single day if stored in a hot enough place, such as a hot car in the sun. Please do a small test with your turquoise before you use it again. If this after-fixing works well, then you know it's okay. I must also mention that the Procion MX type turquoise is more temperature-dependent than the other colors in that series of dyes, so poor turquoise reaction often indicates inadequate warmth in the dye studio. Normally, any temperature over 70°F (21°C) is warm enough if you allow your dye reactions to progress long enough.
Even after Procion MX and other fiber reactive dyes have completely hydrolyzed and are useless for dyeing cotton, they can still be used as acid dyes, on silk, wool, or nylon, if you substitute an acid such as vinegar or citric acid for the soda ash, and apply extra heat. See "Using Fiber reactive dyes on protein fibers". Acid dyes will not work on cotton, however.
(Please help support this web site. Thank you.)
Tuesday, May 27, 2008
tie-dyeing on already black t-shirts
Name:
Russ
Message: I bought 2 black Jeerzee t-shirts along with a tie-die kit at the local craft store. I went through the process and I let them set for 7 hours, with absolutly no change in color to the fabric.what did I do wrong and how can I do it right, please advise, Thank-you Russ
Black is the darkest of all colors, so there is no color you can add on top of it that will make a significant change. Dye is transparent, so it only adds to the color of the shirt underneath; it does not cover it up in any way.
If you want to tie-dye, using any good dye such as
the tie-dye kit you purchased, you must start with light-colored shirts or
fabric. The best and brightest colors require a white background. You can also
get good results by starting with any light color. For example, if you start
with a yellow shirt, and tie-dye it with blue and red, you will end up with a
yellow-background shirt marked with green and orange colors. If you start with a
light blue shirt and dye it with red, violet, and yellow, you will end up with a
blue background with purple, blue-violet, and green. Each of the final colors
includes the original shirt color within it.
as
the tie-dye kit you purchased, you must start with light-colored shirts or
fabric. The best and brightest colors require a white background. You can also
get good results by starting with any light color. For example, if you start
with a yellow shirt, and tie-dye it with blue and red, you will end up with a
yellow-background shirt marked with green and orange colors. If you start with a
light blue shirt and dye it with red, violet, and yellow, you will end up with a
blue background with purple, blue-violet, and green. Each of the final colors
includes the original shirt color within it.
In order to tie-dye your black Jerzee t-shirts, you will have to do a two-step process. In
the first step, use bleach or a
less toxic chemical such as Rit Color Remover to remove color in some
parts of your shirt. In the second step, you will dye the shirt, and the white
streaks left from the first step will produce brilliant colors, surrounded by
the original black in the areas where you did not bleach it. The results of this
two-step process look really great!
In
the first step, use bleach or a
less toxic chemical such as Rit Color Remover to remove color in some
parts of your shirt. In the second step, you will dye the shirt, and the white
streaks left from the first step will produce brilliant colors, surrounded by
the original black in the areas where you did not bleach it. The results of this
two-step process look really great!
To tie-bleach parts of your shirts, tie the shirts as
usual, then soak them in diluted bleach, or put them in a cooking pot with Rit
Color Remover after tying, and gradually bring the temperature to a simmer.
After you have produced the desired white streaks on your shirt in this manner,
immediately wash the shirt. After using chlorine bleach, which
contains hypochlorite, you must neutralize
the bleach to stop it from eating at the fabric, by using Anti-chlor, Bleach
Stop, or hydrogen peroxide (NOT vinegar). There is no need to neutralize
Rit Color Remover.
as
usual, then soak them in diluted bleach, or put them in a cooking pot with Rit
Color Remover after tying, and gradually bring the temperature to a simmer.
After you have produced the desired white streaks on your shirt in this manner,
immediately wash the shirt. After using chlorine bleach, which
contains hypochlorite, you must neutralize
the bleach to stop it from eating at the fabric, by using Anti-chlor, Bleach
Stop, or hydrogen peroxide (NOT vinegar). There is no need to neutralize
Rit Color Remover.
Another good way to make white areas on your black t-shirts is to use any automatic dishwasher detergent that contains black, and paint or stamp it on, or to use a Clorox Bleach Pen to draw designs on the black shirt. Wash, neutralize as above, wash, then dye, and any bleached white areas will seem exceptionally bright after you overdye them.
It's not certain that your Jerzees t-shirts will be suitable for your project. They might not bleach out like you want them to. Not all black dyes will respond to either bleach or color remover, and some respond to one but not the other. I've read that black Jerzees 363 shirts discharge to more of a brown than the desired off-white. You should also never use bleach to discharge any shirt that is not 100% cotton, because hypochlorite will damage all synthetic fibers, such as polyester or spandex.
I know that black Hanes Beefy Tees will bleach out very well, if they haven't changed their dyes. So do black Fruit of the Loom Lofteez shirts. Dharma Trading Company sells several different dischargeable black t-shirts, if you don't have a handy local source, and they probably charge a lot less than your local crafts store does. They are also a good source of another tie-dye kit. You can buy Procion MX dyes for tie-dyeing in over a hundred different colors at Dharma, rather than limiting yourself to the three colors found in most tie-dye kits.
Shirts prepared as described above, by tie-bleaching
and then dyeing, look really great. The black background makes the colored
sections seem brighter, and the dye lasts a long, long time without fading. It
also feels completely oft on the fabric, because you can't feel fabric dye on
the fabric. This is unlike the use of fabric paint.
tie-bleaching
and then dyeing, look really great. The black background makes the colored
sections seem brighter, and the dye lasts a long, long time without fading. It
also feels completely oft on the fabric, because you can't feel fabric dye on
the fabric. This is unlike the use of fabric paint.
Now, most fabric paints are no more suitable to your project than your first attempt to dye with transparent dyes on a black background. Most fabric paints are transparent, like dye, and will not show up on unbleached black. However, there are some fabric paints which are opaque, so that they show up well on a black background. These include the Lumiere line of metallic and pearlescent paints, and the Neopaque line of opaque fabric paints, plus any other fabric paint whose label specifically promises that it is "opaque". Using an opaque or metallic fabric paint, you can paint directly onto a black shirt and produce highly visible results, in just a single step. These results will not feel as soft as dye; often, fabric paint feels a little bit scratchy. Also, the paint will wear off after fewer washings. Follow manufacturer's instructions on heat setting the shirts with a dry iron, one time only, to make the acrylic binder in the paint grab more permanently to the fiber. Always turn fabric-painted items inside-out to launder, and wash on a gentle cycle or hand wash, to slow the inevitable wearing off of the paint.
For more information, please see the following pages....
• Is it possible to tie-dye dark t-shirts with white ?
• How to Tie Dye on Dark Fabric
• Discharged "dyed" Mandalas: no dye added
• What chemicals can be used to remove dye?
• How can I neutralize the damaging effects of chlorine bleach?
(Please help support this web site. Thank you.)
Message: I bought 2 black Jeerzee t-shirts along with a tie-die kit at the local craft store. I went through the process and I let them set for 7 hours, with absolutly no change in color to the fabric.what did I do wrong and how can I do it right, please advise, Thank-you Russ
Black is the darkest of all colors, so there is no color you can add on top of it that will make a significant change. Dye is transparent, so it only adds to the color of the shirt underneath; it does not cover it up in any way.
If you want to tie-dye, using any good dye such
 as
the tie-dye kit you purchased, you must start with light-colored shirts or
fabric. The best and brightest colors require a white background. You can also
get good results by starting with any light color. For example, if you start
with a yellow shirt, and tie-dye it with blue and red, you will end up with a
yellow-background shirt marked with green and orange colors. If you start with a
light blue shirt and dye it with red, violet, and yellow, you will end up with a
blue background with purple, blue-violet, and green. Each of the final colors
includes the original shirt color within it.
as
the tie-dye kit you purchased, you must start with light-colored shirts or
fabric. The best and brightest colors require a white background. You can also
get good results by starting with any light color. For example, if you start
with a yellow shirt, and tie-dye it with blue and red, you will end up with a
yellow-background shirt marked with green and orange colors. If you start with a
light blue shirt and dye it with red, violet, and yellow, you will end up with a
blue background with purple, blue-violet, and green. Each of the final colors
includes the original shirt color within it.In order to tie-dye your black Jerzee t-shirts, you will have to do a two-step process.
 In
the first step, use bleach or a
less toxic chemical such as Rit Color Remover to remove color in some
parts of your shirt. In the second step, you will dye the shirt, and the white
streaks left from the first step will produce brilliant colors, surrounded by
the original black in the areas where you did not bleach it. The results of this
two-step process look really great!
In
the first step, use bleach or a
less toxic chemical such as Rit Color Remover to remove color in some
parts of your shirt. In the second step, you will dye the shirt, and the white
streaks left from the first step will produce brilliant colors, surrounded by
the original black in the areas where you did not bleach it. The results of this
two-step process look really great!To tie-bleach parts of your shirts, tie the shirts
 as
usual, then soak them in diluted bleach, or put them in a cooking pot with Rit
Color Remover after tying, and gradually bring the temperature to a simmer.
After you have produced the desired white streaks on your shirt in this manner,
immediately wash the shirt. After using chlorine bleach, which
contains hypochlorite, you must neutralize
the bleach to stop it from eating at the fabric, by using Anti-chlor, Bleach
Stop, or hydrogen peroxide (NOT vinegar). There is no need to neutralize
Rit Color Remover.
as
usual, then soak them in diluted bleach, or put them in a cooking pot with Rit
Color Remover after tying, and gradually bring the temperature to a simmer.
After you have produced the desired white streaks on your shirt in this manner,
immediately wash the shirt. After using chlorine bleach, which
contains hypochlorite, you must neutralize
the bleach to stop it from eating at the fabric, by using Anti-chlor, Bleach
Stop, or hydrogen peroxide (NOT vinegar). There is no need to neutralize
Rit Color Remover.Another good way to make white areas on your black t-shirts is to use any automatic dishwasher detergent that contains black, and paint or stamp it on, or to use a Clorox Bleach Pen to draw designs on the black shirt. Wash, neutralize as above, wash, then dye, and any bleached white areas will seem exceptionally bright after you overdye them.
It's not certain that your Jerzees t-shirts will be suitable for your project. They might not bleach out like you want them to. Not all black dyes will respond to either bleach or color remover, and some respond to one but not the other. I've read that black Jerzees 363 shirts discharge to more of a brown than the desired off-white. You should also never use bleach to discharge any shirt that is not 100% cotton, because hypochlorite will damage all synthetic fibers, such as polyester or spandex.
I know that black Hanes Beefy Tees will bleach out very well, if they haven't changed their dyes. So do black Fruit of the Loom Lofteez shirts. Dharma Trading Company sells several different dischargeable black t-shirts, if you don't have a handy local source, and they probably charge a lot less than your local crafts store does. They are also a good source of another tie-dye kit. You can buy Procion MX dyes for tie-dyeing in over a hundred different colors at Dharma, rather than limiting yourself to the three colors found in most tie-dye kits.
Shirts prepared as described above, by
 tie-bleaching
and then dyeing, look really great. The black background makes the colored
sections seem brighter, and the dye lasts a long, long time without fading. It
also feels completely oft on the fabric, because you can't feel fabric dye on
the fabric. This is unlike the use of fabric paint.
tie-bleaching
and then dyeing, look really great. The black background makes the colored
sections seem brighter, and the dye lasts a long, long time without fading. It
also feels completely oft on the fabric, because you can't feel fabric dye on
the fabric. This is unlike the use of fabric paint. Now, most fabric paints are no more suitable to your project than your first attempt to dye with transparent dyes on a black background. Most fabric paints are transparent, like dye, and will not show up on unbleached black. However, there are some fabric paints which are opaque, so that they show up well on a black background. These include the Lumiere line of metallic and pearlescent paints, and the Neopaque line of opaque fabric paints, plus any other fabric paint whose label specifically promises that it is "opaque". Using an opaque or metallic fabric paint, you can paint directly onto a black shirt and produce highly visible results, in just a single step. These results will not feel as soft as dye; often, fabric paint feels a little bit scratchy. Also, the paint will wear off after fewer washings. Follow manufacturer's instructions on heat setting the shirts with a dry iron, one time only, to make the acrylic binder in the paint grab more permanently to the fiber. Always turn fabric-painted items inside-out to launder, and wash on a gentle cycle or hand wash, to slow the inevitable wearing off of the paint.
For more information, please see the following pages....
• Is it possible to tie-dye dark t-shirts with white ?
• How to Tie Dye on Dark Fabric
• Discharged "dyed" Mandalas: no dye added
• What chemicals can be used to remove dye?
• How can I neutralize the damaging effects of chlorine bleach?
(Please help support this web site. Thank you.)
Monday, May 26, 2008
steaming dry silk with food dyes
Name:
Elizabeth
Message: your website is wonderful -
my question is -
i have dyed some very small pieces of silk chiffon with food coloring. they will eventually end up as part of a mixed media piece, framed under glass. -- all the dyed pieces of silk are dry now so - regarding their lightfastness: is it too late to steam them? will it help at all? and if so, must they be wrapped in plastic for a stovetop steamer, or can they merely be placed on it? will ironing help at all? or hurt? or do nothing?
i have combed over your site, and parts of this question are dealt with but not, that i can find, all put together as such & pertinent to food color on silk. please pardon any redundancy if it is the case.
It's not too late to steam your silk. In fact, the most usual way to steam dyed silk is to let it dry, and then steam at a convenient time.
Do not wrap dry silk in plastic wrap for steaming: plastic wrap is suitable only for fully wet fabric or yarn. Moisture in some form is required for any dye to bond to the natural fiber. Instead, wrap your dry silk in layers of unprinted newsprint paper. The silk is protected from other parts of the silk by layers of paper. For pieces of silk that are too large to fit in the steamer pot as it is, roll the paper up with the silk inside, then coil the paper roll, to make a shape that will fit in your steamer. Steam for twenty to thirty minutes, unless instructed otherwise.
Unfortunately, while steaming will help with washfastness, I am not sure how much good it will do for the lightfastness of the pieces. It is a good idea to do the steaming, since properly fixed dye is in general more light-resisatnat than dye that has not been fixed to the fiber. Food coloring is not particularly noted for being resistant to fading from light. I am sure that ironing will make no appreciable difference in the lightfastness of any dye, since dry heat does not encourage dye to bond with the fiber; dry heat is used only for acrylic-based fabric paints. Framing under glass will help a little, especially if the glass is a little tinted, so that it reduces the amount of visible light that reaches the fabric. Ultraviolet protection sprays will not help at all, from my observations.
The most light-resistant of dyes are the vat dyes, including the light-developed Inkodyes sold by Dharma Trading Company. Pigments are in many cases more lightfast than dyes, so fabric paint is in some cases a better choice than fabric dye, when items will be exposed to light but rarely if ever washed. Some acid dyes are acceptably lightfast, while others are not. Food dyes are a type of acid dye. I'm sure you have already looked at my page on Lightfastness of Different Types of Dyes, which, when you wrote, did not include ratings for food colorings. (I will be adding them.) Lightfastness ratings for acid dyes range from 1 (very poor indeed) to 7 (quite lightfast), on a scale of 1 to 8. Published numbers for the lightfastness of food colors range from 3, which is poor, to 6, which is acceptable. It is unknown how these values will shift when the dyes are applied to silk. Some dyes, such as Procion blue MX-7RX, are more lightfast when they have been applied to silk.
All Colour Supplies Pty Ltd of Australia claims the following lightfastness numbers for food colorings:
Note that lightfastness may be significantly altered for dyes that have been attached to textile fibers, and that the lightfastness may be very different on oen fiber than another; however, we have no information about how the lightfastness of food coloring smay change once they have been binded to silk or any other textile fiber.
As you can see, under the conditions used for testing by the All Colour Supplies company, Tartrazine (FD&C yellow 5) is a better choice, for light-fastness, than Sunset Yellow (FD&C yellow 6); Allura Red (FD&C red 40) is a better choice than Erythrosine ((FD&C red 3); and Brilliant Blue (FD&C blue 2) is a better choice than Indigo Carmine (FD&C blue 1). A lightfastness of 5, on a scale of 1 to 8, is ranked as having "fair" lightfastness, and according to the ASTM lightfastness standards (see the chart on Bruce McAvoy's site) "will remain unchanged for 15 to 50 years with proper mounting and display." ("May be satisfactory when used full strength or with extra protection from exposure to light.") Tartrazine's lightfastness rating of 6 "will remain unchanged for 50 to 100 years of light exposure with proper mounting and display."
As it happens, each of the better colors of these three pairs are the ones more commonly found in food colorings, so you may have used the more light-fast dyes without knowing about their lightfastness. Indigo Carmine is highly susceptible to damage from heat, so it probably should not be steamed, if you used it. I have found it more difficult to find Indigo Carmine, however, so you probably did not use it.
Among natural food colorings, lightfastness is very poor, poorer than that for synthetic food colorings. Cochineal is used in the artists' pigment carmine; Bruce MacEvoy rates it as completely unacceptable for painting due to its poor lightfastness. Turmeric, a spice that is used as a natural yellow food coloring, is noted for poor lightfastness, as well, as are most natural yellow dyes.
(Please help support this web site. Thank you.)
Message: your website is wonderful -
my question is -
i have dyed some very small pieces of silk chiffon with food coloring. they will eventually end up as part of a mixed media piece, framed under glass. -- all the dyed pieces of silk are dry now so - regarding their lightfastness: is it too late to steam them? will it help at all? and if so, must they be wrapped in plastic for a stovetop steamer, or can they merely be placed on it? will ironing help at all? or hurt? or do nothing?
i have combed over your site, and parts of this question are dealt with but not, that i can find, all put together as such & pertinent to food color on silk. please pardon any redundancy if it is the case.
It's not too late to steam your silk. In fact, the most usual way to steam dyed silk is to let it dry, and then steam at a convenient time.
Do not wrap dry silk in plastic wrap for steaming: plastic wrap is suitable only for fully wet fabric or yarn. Moisture in some form is required for any dye to bond to the natural fiber. Instead, wrap your dry silk in layers of unprinted newsprint paper. The silk is protected from other parts of the silk by layers of paper. For pieces of silk that are too large to fit in the steamer pot as it is, roll the paper up with the silk inside, then coil the paper roll, to make a shape that will fit in your steamer. Steam for twenty to thirty minutes, unless instructed otherwise.
Unfortunately, while steaming will help with washfastness, I am not sure how much good it will do for the lightfastness of the pieces. It is a good idea to do the steaming, since properly fixed dye is in general more light-resisatnat than dye that has not been fixed to the fiber. Food coloring is not particularly noted for being resistant to fading from light. I am sure that ironing will make no appreciable difference in the lightfastness of any dye, since dry heat does not encourage dye to bond with the fiber; dry heat is used only for acrylic-based fabric paints. Framing under glass will help a little, especially if the glass is a little tinted, so that it reduces the amount of visible light that reaches the fabric. Ultraviolet protection sprays will not help at all, from my observations.
The most light-resistant of dyes are the vat dyes, including the light-developed Inkodyes sold by Dharma Trading Company. Pigments are in many cases more lightfast than dyes, so fabric paint is in some cases a better choice than fabric dye, when items will be exposed to light but rarely if ever washed. Some acid dyes are acceptably lightfast, while others are not. Food dyes are a type of acid dye. I'm sure you have already looked at my page on Lightfastness of Different Types of Dyes, which, when you wrote, did not include ratings for food colorings. (I will be adding them.) Lightfastness ratings for acid dyes range from 1 (very poor indeed) to 7 (quite lightfast), on a scale of 1 to 8. Published numbers for the lightfastness of food colors range from 3, which is poor, to 6, which is acceptable. It is unknown how these values will shift when the dyes are applied to silk. Some dyes, such as Procion blue MX-7RX, are more lightfast when they have been applied to silk.
All Colour Supplies Pty Ltd of Australia claims the following lightfastness numbers for food colorings:
| name | CI # | CI name | FD & C | E. Code | Class | Light Stability |
|---|---|---|---|---|---|---|
| Tartrazine | 19140 | CI food yellow 4 | FD & C yellow 5 | E102 | Monoazo | 6 |
| Sunset Yellow | 15985 | CI food yellow 3 | FD & C yellow 6 | E110 | Monoazo | 4 |
| Erythrosine | 45430 | CI food red 14 | FD & C red No.3 | E127 | Xanthene | 3 |
| Allura Red | 16035 | CI food red 17 | FD & C Red No.40 | E129 | Monoazo | 5 |
| Brilliant Blue | 42090 | CI food blue 2 | FD & C Blue No.1 | E133 | Triaryl- methane | 5 | 73015 | CI food blue 1 | FD & C Blue No.2 | E132 | Indigoid | 3 |
Note that lightfastness may be significantly altered for dyes that have been attached to textile fibers, and that the lightfastness may be very different on oen fiber than another; however, we have no information about how the lightfastness of food coloring smay change once they have been binded to silk or any other textile fiber.
As you can see, under the conditions used for testing by the All Colour Supplies company, Tartrazine (FD&C yellow 5) is a better choice, for light-fastness, than Sunset Yellow (FD&C yellow 6); Allura Red (FD&C red 40) is a better choice than Erythrosine ((FD&C red 3); and Brilliant Blue (FD&C blue 2) is a better choice than Indigo Carmine (FD&C blue 1). A lightfastness of 5, on a scale of 1 to 8, is ranked as having "fair" lightfastness, and according to the ASTM lightfastness standards (see the chart on Bruce McAvoy's site) "will remain unchanged for 15 to 50 years with proper mounting and display." ("May be satisfactory when used full strength or with extra protection from exposure to light.") Tartrazine's lightfastness rating of 6 "will remain unchanged for 50 to 100 years of light exposure with proper mounting and display."
As it happens, each of the better colors of these three pairs are the ones more commonly found in food colorings, so you may have used the more light-fast dyes without knowing about their lightfastness. Indigo Carmine is highly susceptible to damage from heat, so it probably should not be steamed, if you used it. I have found it more difficult to find Indigo Carmine, however, so you probably did not use it.
Among natural food colorings, lightfastness is very poor, poorer than that for synthetic food colorings. Cochineal is used in the artists' pigment carmine; Bruce MacEvoy rates it as completely unacceptable for painting due to its poor lightfastness. Turmeric, a spice that is used as a natural yellow food coloring, is noted for poor lightfastness, as well, as are most natural yellow dyes.
(Please help support this web site. Thank you.)
Sunday, May 25, 2008
Is it possible to tie-dye black t-shirts with white?
Name:
Lia
Rit Color Remover Removes Dyes

Message: Hello, I have some black team shirts that have been silk screened on one side. I'd like to tie dye some of them with white... is this possible?
Yes, it is possible. You can try discharge dyeing, which means making designs by removing dye, with either chlorine bleach (hypochlorite) or a safer and gentler chemical such as Rit Color Remover or Jacquard Discharge Paste, or you can use an opaque white fabric paint, such as Neopaque.
Not all black dyes can be discharged. Some will stay black no matter what you do. You should take one of the shirts (was there one that did not print well?) and test your first choice of discharge agent on it, in as inconspicuous a place as possible. If you want to use chlorine bleach, a handy way to do the test is to use a Clorox Bleach Pen. Rit Color Remover and Jacquard Discharge Paste will give similar results to each other, but they may be different from the results produced by chlorine bleach.
For more information, see:
• How to Tie Dye on Dark Fabric
• What chemicals can be used to remove dye?
• How can I neutralize the damaging effects of chlorine bleach?
As a general rule, screen printing is not usually disturbed by dyeing or discharging, but changing the color of the fabric behind the screen print may make it harder to read. Always do one test garment before treating a large number of them.
(Please help support this web site. Thank you.)
—ADVERTISEMENTS—
Bleach PenRit Color Remover Removes Dyes
Message: Hello, I have some black team shirts that have been silk screened on one side. I'd like to tie dye some of them with white... is this possible?
Yes, it is possible. You can try discharge dyeing, which means making designs by removing dye, with either chlorine bleach (hypochlorite) or a safer and gentler chemical such as Rit Color Remover or Jacquard Discharge Paste, or you can use an opaque white fabric paint, such as Neopaque.
Not all black dyes can be discharged. Some will stay black no matter what you do. You should take one of the shirts (was there one that did not print well?) and test your first choice of discharge agent on it, in as inconspicuous a place as possible. If you want to use chlorine bleach, a handy way to do the test is to use a Clorox Bleach Pen. Rit Color Remover and Jacquard Discharge Paste will give similar results to each other, but they may be different from the results produced by chlorine bleach.
For more information, see:
• How to Tie Dye on Dark Fabric
• What chemicals can be used to remove dye?
• How can I neutralize the damaging effects of chlorine bleach?
As a general rule, screen printing is not usually disturbed by dyeing or discharging, but changing the color of the fabric behind the screen print may make it harder to read. Always do one test garment before treating a large number of them.
(Please help support this web site. Thank you.)
Thursday, May 22, 2008
I really need to know how to tie the mirror spiral shirt
Name:
Sarah
Message: Hi i'm Sarah. I'm doing like 7 shirts for my school team because were having a athletic olympic thing where we compete against other teams... and were doing tie dye of course and i really needed to know how to tie the mirror spiral shirt with turquoise and purple. Because i need really need to see like a video of it because just the words on how to say it really confuses me. If you could send me a video by sunday or something that would help at all that would be great....Please and thankyou....SARAH:)
There is a good video on how to do a single spiral embedded in my "How to Tie Dye a Spiral" page. All you have to do for a mirror spiral is to fold your shirt in half before doing what is shown on the video.
I'm afraid that I can't create a custom video on how to do a mirror spiral. You can try searching on a video clip website, but not all of the how-to videos you can find are reliable. Some will point you in the wrong direction and waste your time and effort.
If you find these instructions to be too confusing, you should buy a good DVD on how to do it. The Tie Dye 101 DVD by True Tie Dye makes it easy to learn how to do spirals and other tie-dye techniques. Their other DVD set, Advanced Tie Dye techniques, covers hearts, peace signs, stars, arrowheads, mandalas, sunbursts, and other advanced designs.
Alternatively, you can just do trial and error, as most people do. Just fold your shirt in half, and tie a standard spiral. If you are not sure what the instructions are saying, make your best guess, and try it. See what you get. Next time, do it a little differently. Eventually, you will learn how to do exactly what you want. Hands-on is the best way to learn.
If you use good materials, your beginner's attempts will still look good. Use a good cool-water tie-dye kit, or else buy the ingredients separately, Procion MX dyes plus soda ash plus urea. (Do NOT try to tie-dye using a hot-water dye such as Rit dye, because the colors will fade very quickly and bleed badly in the laundry.)
Here are some pages you should look at:
• How do you tie-dye a spiral pattern?
• How to Tie Dye - Complete Instructions
• Double Spiral at the Tie Dye Wiki
• Single Spiral at the Tie Dye Wiki
• True Tie Dye videos (commercial site)
(Please help support this web site. Thank you.)
—ADVERTISEMENTS—
DVDs on How to Tie Dye—ADVERTISEMENTS—
Tie Dye KitsMessage: Hi i'm Sarah. I'm doing like 7 shirts for my school team because were having a athletic olympic thing where we compete against other teams... and were doing tie dye of course and i really needed to know how to tie the mirror spiral shirt with turquoise and purple. Because i need really need to see like a video of it because just the words on how to say it really confuses me. If you could send me a video by sunday or something that would help at all that would be great....Please and thankyou....SARAH:)
There is a good video on how to do a single spiral embedded in my "How to Tie Dye a Spiral" page. All you have to do for a mirror spiral is to fold your shirt in half before doing what is shown on the video.
I'm afraid that I can't create a custom video on how to do a mirror spiral. You can try searching on a video clip website, but not all of the how-to videos you can find are reliable. Some will point you in the wrong direction and waste your time and effort.
If you find these instructions to be too confusing, you should buy a good DVD on how to do it. The Tie Dye 101 DVD by True Tie Dye makes it easy to learn how to do spirals and other tie-dye techniques. Their other DVD set, Advanced Tie Dye techniques, covers hearts, peace signs, stars, arrowheads, mandalas, sunbursts, and other advanced designs.
Alternatively, you can just do trial and error, as most people do. Just fold your shirt in half, and tie a standard spiral. If you are not sure what the instructions are saying, make your best guess, and try it. See what you get. Next time, do it a little differently. Eventually, you will learn how to do exactly what you want. Hands-on is the best way to learn.
If you use good materials, your beginner's attempts will still look good. Use a good cool-water tie-dye kit, or else buy the ingredients separately, Procion MX dyes plus soda ash plus urea. (Do NOT try to tie-dye using a hot-water dye such as Rit dye, because the colors will fade very quickly and bleed badly in the laundry.)
Here are some pages you should look at:
• How do you tie-dye a spiral pattern?
• How to Tie Dye - Complete Instructions
• Double Spiral at the Tie Dye Wiki
• Single Spiral at the Tie Dye Wiki
• True Tie Dye videos (commercial site)
(Please help support this web site. Thank you.)
Wednesday, May 21, 2008
Prior to overdyeing, do I resoak these fabric pieces in soda ash once again?
Name: Diana
Message: I dyed some fabric today and would like the colors to be more intense and even. Prior to overdyeing, do I resoak these fabric pieces in soda ash once again? Thanks
Yes. Before you can tell whether your dyed items are deeply colored enough, you have to rinse and dry them. This removes the soda ash you used in the first dyeing, so you must apply more. Soda ash is very easily removed by rinsing.
Overdyeing is a great way to get deeper, richer colors. It appears that additional dye sites are opened up by washing and drying, by physically changing the orientation of the fibers slightly, so you can get richer colors by dyeing fabric two or more times than you can get by imply using more dye in a single step.
For greater evenness in color, try using more water, relative to the amount of fabric, and stir the fabric in the dye more frequently. In low water immersion dyeing, for smoother color gradations, try adding the soda ash after the dye, rather than presoaking it as in tie-dyeing.
For dyeing a perfectly smooth solid color, use three gallons of water per pound of fabric, stir the fabric with the dye for a while (say twenty minutes) before adding soda ash, then add the soda ash in three parts, stirring constantly, and then stir for half an hour to an hour after all of the soda ash is added. This is the same method as that used for dyeing in the washing machine.
(Please help support this web site. Thank you.)
Message: I dyed some fabric today and would like the colors to be more intense and even. Prior to overdyeing, do I resoak these fabric pieces in soda ash once again? Thanks
Yes. Before you can tell whether your dyed items are deeply colored enough, you have to rinse and dry them. This removes the soda ash you used in the first dyeing, so you must apply more. Soda ash is very easily removed by rinsing.
Overdyeing is a great way to get deeper, richer colors. It appears that additional dye sites are opened up by washing and drying, by physically changing the orientation of the fibers slightly, so you can get richer colors by dyeing fabric two or more times than you can get by imply using more dye in a single step.
For greater evenness in color, try using more water, relative to the amount of fabric, and stir the fabric in the dye more frequently. In low water immersion dyeing, for smoother color gradations, try adding the soda ash after the dye, rather than presoaking it as in tie-dyeing.
For dyeing a perfectly smooth solid color, use three gallons of water per pound of fabric, stir the fabric with the dye for a while (say twenty minutes) before adding soda ash, then add the soda ash in three parts, stirring constantly, and then stir for half an hour to an hour after all of the soda ash is added. This is the same method as that used for dyeing in the washing machine.
(Please help support this web site. Thank you.)
Tuesday, May 20, 2008
How long can you store dye premix solution that hasn't been mixed with any dye?
Name:
Kim
Message: I was wondering for dyeing with procion mx dyes, how long can you store dye premix solution that hasn't been mixed with any dye? The solution being a mix of water, water softener, urea, and kelp.
This premix solution, also referred to by many dyers as "chemical water", will usually stay good for days at room temperature, possibly even months in some cases. It is safe to continue to use it as long as there is no smell of ammonia. If you detect the smell of ammonia from your solutions, this means that the urea has begun breaking down. Because ammonia alters the pH of dye solutions, you should not use any such mixtures after the urea has started to decompose to ammonia.
It is possible to have mold growth in your premix solution. This could have unpredictable effects on pH. If you see any sign of cloudiness, discard it and make up fresh solution.
Refrigeration should help the mixture to stay good much longer. The water softener (sodium hexametaphosphate) helps keep the alginate in the kelp from gelling.
(Please help support this web site. Thank you.)
Message: I was wondering for dyeing with procion mx dyes, how long can you store dye premix solution that hasn't been mixed with any dye? The solution being a mix of water, water softener, urea, and kelp.
This premix solution, also referred to by many dyers as "chemical water", will usually stay good for days at room temperature, possibly even months in some cases. It is safe to continue to use it as long as there is no smell of ammonia. If you detect the smell of ammonia from your solutions, this means that the urea has begun breaking down. Because ammonia alters the pH of dye solutions, you should not use any such mixtures after the urea has started to decompose to ammonia.
It is possible to have mold growth in your premix solution. This could have unpredictable effects on pH. If you see any sign of cloudiness, discard it and make up fresh solution.
Refrigeration should help the mixture to stay good much longer. The water softener (sodium hexametaphosphate) helps keep the alginate in the kelp from gelling.
(Please help support this web site. Thank you.)
Monday, May 19, 2008
How long can you keep procion MX dyes, after you mix them with water and urea?
Message: How long can you keep Procion MX dyes, after you mix them
with water and
urea?
Procion MX are the fastest-reacting of all of the fiber reactive dyes. This means that you can use them at room temperature, anything over 70°F, without having to apply additional heat, but it also means that they spoil more quickly.
As a general rule, you can store Procion MX dye mixtures at room temperature for a week or two without problems, as long as not even a tiny drop of soda ash has gotten into any of them. (They will spoil within an hour or two if you get soda ash in them, even if you just dip a fine paintbrush into the dye repeatedly to apply it to a dry soda-ash-soaked cloth.) Temperature has a big effect on how quickly the dyes will spoil. If you refrigerate your dye mixtures, they can stay good longer, up to a couple of months (and in some cases longer). The strength of the dye mixtures will gradually decline, for use on cotton, as the dye reacts with the water. You cannot tell whether the dye has spoiled by looking at it; before any large or important project, if your dye solutions are old, it's a good idea to do a small test to see if the dye will bond to cotton fabric.
The different Procion MX dyes have differing reactivities, so some spoil faster than others. Fuchsia, or red MX-8B, is the fastest-reacting of the dichlorotriazine dyes, and therefore it spoils the most quickly. Turquoise, or turquoise MX-G, is the slowest-to-react of these dyes, so it should last the longest. However, turquoise is a special case, in the the hue of the dye mixtures gradually becomes more blue and less green with time. This is because the copper phthalocyanine structures stack up together, forming larger dye particles that absorb light differently. See "A Beautiful Blue: Procion Turquoise MX-G", for more information about this phenomenon.
Even when Procion MX has completely spoiled for use on cotton, it can still be used as an acid dye. Acid dyes do not work on cotton or rayon, but they work well on silk, wool, and nylon. Instead of using soda ash as an auxiliary chemical, you would use an acid such as vinegar. See "Fiber reactive dyes on protein fibers".
If you detect the smell of ammonia from your dye mixtures, this means that the urea has begun breaking down. Because ammonia alters the pH of dye solutions, you should not use any dye mixtures after the urea has started to decompose to ammonia. At that point, they are no longer useful as reactive dyes or as acid dyes.
(Please help support this web site. Thank you.)
Procion MX are the fastest-reacting of all of the fiber reactive dyes. This means that you can use them at room temperature, anything over 70°F, without having to apply additional heat, but it also means that they spoil more quickly.
As a general rule, you can store Procion MX dye mixtures at room temperature for a week or two without problems, as long as not even a tiny drop of soda ash has gotten into any of them. (They will spoil within an hour or two if you get soda ash in them, even if you just dip a fine paintbrush into the dye repeatedly to apply it to a dry soda-ash-soaked cloth.) Temperature has a big effect on how quickly the dyes will spoil. If you refrigerate your dye mixtures, they can stay good longer, up to a couple of months (and in some cases longer). The strength of the dye mixtures will gradually decline, for use on cotton, as the dye reacts with the water. You cannot tell whether the dye has spoiled by looking at it; before any large or important project, if your dye solutions are old, it's a good idea to do a small test to see if the dye will bond to cotton fabric.
The different Procion MX dyes have differing reactivities, so some spoil faster than others. Fuchsia, or red MX-8B, is the fastest-reacting of the dichlorotriazine dyes, and therefore it spoils the most quickly. Turquoise, or turquoise MX-G, is the slowest-to-react of these dyes, so it should last the longest. However, turquoise is a special case, in the the hue of the dye mixtures gradually becomes more blue and less green with time. This is because the copper phthalocyanine structures stack up together, forming larger dye particles that absorb light differently. See "A Beautiful Blue: Procion Turquoise MX-G", for more information about this phenomenon.
Even when Procion MX has completely spoiled for use on cotton, it can still be used as an acid dye. Acid dyes do not work on cotton or rayon, but they work well on silk, wool, and nylon. Instead of using soda ash as an auxiliary chemical, you would use an acid such as vinegar. See "Fiber reactive dyes on protein fibers".
If you detect the smell of ammonia from your dye mixtures, this means that the urea has begun breaking down. Because ammonia alters the pH of dye solutions, you should not use any dye mixtures after the urea has started to decompose to ammonia. At that point, they are no longer useful as reactive dyes or as acid dyes.
(Please help support this web site. Thank you.)
Sunday, May 18, 2008
neutralizing sodium hypochlorite with sodium metabifulfite
Name:
Brent
Thiosulfate (Hypo)
Sodium sulfite
Message: After cleaning a moldy exterior wood deck with 12.5% sodium hypochlorite, surfactants, detergent and water (about 3 to 1 ratio) I would like to neutralize the sodium hypochlorite. How much sodium metabisulfite per gallon do you recommend as a spray on rinse?
I've never used a neutralizing solution on bleach-treated wood, but you might do well to use the standard strength that is used for neutralizing bleach in fabric, which is two teaspoons (4.4 grams) in five gallons of water. Be sure that you have rinsed well with plain water before applying the metabisulfite.
Depending on how much of the bleach you used, you should be able to calculate how much metabisulfite will be needed, in total. If one sodium hypochlorite molecule (molecular weight 74.44) reacts with one molecule of sodium metabisulfite (molecular weight 190.1), you'll need two and a half grams of metabisulfite for every gram of hypochlorite.
from fumes
One quart of 12.5% sodium hypochlorite contains 125 grams of sodium hypochlorite, which should require 320 grams of sodium metabisulfite to neutralize it, or a little less than a pound, if these calculations are correct.
It is important to note that the fumes produced by sodium metabisulfite include sulfur dioxide, a very irritating gas that can cause dangerous reactions in people who have asthma. You can protect your lungs by wearing a cartridge respirator fitted with an acid gas cartridge.
For more information, see "How can I neutralize the damaging effects of chlorine bleach?", from "Frequently Asked Questions About Dyeing".
(Please help support this web site. Thank you.)
—ADVERTISEMENTS—
Anti-chlor chemicals for neutralizing bleachThiosulfate (Hypo)
Sodium sulfite
Message: After cleaning a moldy exterior wood deck with 12.5% sodium hypochlorite, surfactants, detergent and water (about 3 to 1 ratio) I would like to neutralize the sodium hypochlorite. How much sodium metabisulfite per gallon do you recommend as a spray on rinse?
I've never used a neutralizing solution on bleach-treated wood, but you might do well to use the standard strength that is used for neutralizing bleach in fabric, which is two teaspoons (4.4 grams) in five gallons of water. Be sure that you have rinsed well with plain water before applying the metabisulfite.
Depending on how much of the bleach you used, you should be able to calculate how much metabisulfite will be needed, in total. If one sodium hypochlorite molecule (molecular weight 74.44) reacts with one molecule of sodium metabisulfite (molecular weight 190.1), you'll need two and a half grams of metabisulfite for every gram of hypochlorite.
—ADVERTISEMENT—
Protect yourselffrom fumes
One quart of 12.5% sodium hypochlorite contains 125 grams of sodium hypochlorite, which should require 320 grams of sodium metabisulfite to neutralize it, or a little less than a pound, if these calculations are correct.
It is important to note that the fumes produced by sodium metabisulfite include sulfur dioxide, a very irritating gas that can cause dangerous reactions in people who have asthma. You can protect your lungs by wearing a cartridge respirator fitted with an acid gas cartridge.
For more information, see "How can I neutralize the damaging effects of chlorine bleach?", from "Frequently Asked Questions About Dyeing".
(Please help support this web site. Thank you.)
Saturday, May 17, 2008
Although the linen has dyed successfully the stitching has not taken the colour and they don't look any good!
Name:
Jacquie
Message: I have dyed a pair of linen trousers (dark brown), using Dylon. Although the linen has dyed successfully the stitching has not taken the colour and they don't look any good! Any suggestions as to what I can use?
This is a common problem. See, in my FAQ, the question, "Why did the thread stay white when I dyed clothing?".
The problem is that your 100% linen trousers were sewn together with 100% polyester thread, which is almost undyeable. See "Dyeing Polyester with Disperse Dyes". This is true of just about all commercially-sewn garments, unless they are marked PFD (for "Prepared For Dyeing"), RTD (for "Ready To Dye" or specifically "sewn with cotton thread". You will not find such garments at any ordinary store, but you can find them at wholesalers that supply garments to custom printing or garment-dyeing shops, and you can also buy them from a limited number of blank clothing suppliers, such as Dharma Trading Company.
In my experience, fabric markers don't do a good enough job on polyester thread to be worth trying. It's a lot of trouble to apply, but the polyester thread takes the fabric marker very lightly, so it still contrasts markedly with the natural fiber fabric.
Dyeing all of the thread at once by immersing the whole garment in polyester dye and boiling for an hour is far too much trouble: for one thing, it'll take an expensive large non-aluminum cooking pot which should never be reused for food. For another, the hour's worth of boiling is apt to damage the trousers.
I think that it would be too much trouble to iron-transfer disperse dye to all of the stitching, but this is an option to consider. Disperse dye will color polyester brilliantly, but will eventually wash out of natural fibers such as cotton or linen. To do this, buy disperse dye Fabric Transfer Crayons or disperse dye transfer paint, and apply it to several sheets of paper. Cut the paper into thin strips and iron it on to all of the polyester stitching, one bit at a time. This is easier than using fabric paint, because you don't have to wait a day for the paint to dry on one section, before you move on to the next, and the results will probably be better.
Disperse dye fabric transfer crayons are readily found in crafts stores and sewing stores. Disperse dye transfer paints can be purchased from several mail-order sources, including Omega Dyes in the UK, Zijdelings in the Netherlands, The Thread Studio in Australia, and G&S Dye in Canada. In the US, purchase disperse dye powder from PRO Chemical & Dye in Massachusetts and use it to make your own transfer paint. See my page listing Sources for Dyeing Supplies Around the World for contact information for these and other suppliers.
(Please help support this web site. Thank you.)
Message: I have dyed a pair of linen trousers (dark brown), using Dylon. Although the linen has dyed successfully the stitching has not taken the colour and they don't look any good! Any suggestions as to what I can use?
This is a common problem. See, in my FAQ, the question, "Why did the thread stay white when I dyed clothing?".
The problem is that your 100% linen trousers were sewn together with 100% polyester thread, which is almost undyeable. See "Dyeing Polyester with Disperse Dyes". This is true of just about all commercially-sewn garments, unless they are marked PFD (for "Prepared For Dyeing"), RTD (for "Ready To Dye" or specifically "sewn with cotton thread". You will not find such garments at any ordinary store, but you can find them at wholesalers that supply garments to custom printing or garment-dyeing shops, and you can also buy them from a limited number of blank clothing suppliers, such as Dharma Trading Company.
In my experience, fabric markers don't do a good enough job on polyester thread to be worth trying. It's a lot of trouble to apply, but the polyester thread takes the fabric marker very lightly, so it still contrasts markedly with the natural fiber fabric.
Dyeing all of the thread at once by immersing the whole garment in polyester dye and boiling for an hour is far too much trouble: for one thing, it'll take an expensive large non-aluminum cooking pot which should never be reused for food. For another, the hour's worth of boiling is apt to damage the trousers.
I think that it would be too much trouble to iron-transfer disperse dye to all of the stitching, but this is an option to consider. Disperse dye will color polyester brilliantly, but will eventually wash out of natural fibers such as cotton or linen. To do this, buy disperse dye Fabric Transfer Crayons or disperse dye transfer paint, and apply it to several sheets of paper. Cut the paper into thin strips and iron it on to all of the polyester stitching, one bit at a time. This is easier than using fabric paint, because you don't have to wait a day for the paint to dry on one section, before you move on to the next, and the results will probably be better.
Disperse dye fabric transfer crayons are readily found in crafts stores and sewing stores. Disperse dye transfer paints can be purchased from several mail-order sources, including Omega Dyes in the UK, Zijdelings in the Netherlands, The Thread Studio in Australia, and G&S Dye in Canada. In the US, purchase disperse dye powder from PRO Chemical & Dye in Massachusetts and use it to make your own transfer paint. See my page listing Sources for Dyeing Supplies Around the World for contact information for these and other suppliers.
(Please help support this web site. Thank you.)
Friday, May 16, 2008
Dyeing variegated wool with Lanaset dyes
Name:
Marion
Message: My guild showed me how to dye wool by simmering in white vinegar then putting in a pan of water and sprinkling dye powder on top of the water and covering with foil to form steam which activated the dye. The result was varigated wool. I want to buy the Lanaset dye to do this. Please advise which ones and how much. Thank you.
You can buy Lanaset dyes in some knitting shops, or by mail order from several good dye suppliers. See my blog entry, "Who sells Lanaset dyes?". Paradise Fibers and PRO Chemical & Dye sell a four-color sampler kit which is ideal for beginners.
There will be some instructions that come with the dyes. You can also look at ProChem's instructions for "Rainbow Dyeing using Sabraset/Lanaset Dyes", and at Maiwa's Lanaset-Telana Datasheet [PDF]. Books with instructions for using Lanaset dye include Deb Menz's Color In Spinning, Dagmar Klos's Dyer's Companion, Lynne Vogel's The Twisted Sisters Sock Workbook: Dyeing, Painting, Spinning, Designing, Knitting, and Karren Brito's Shibori: Creating Color And Texture on Silk.
Lanaset dye is the most washfast of all wool dyes, when heat-set for the full recommended period of time, either by immersing the wool and the dye in simmering water for an hour or so, or by steaming the wool over boiling water in a steamer for twenty minutes or so. Unlike other dyes used for wool, it is washfast even in hot water.
(Please help support this web site. Thank you.)
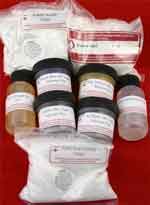
(For silk, wool, angora, mohair & nylon)
Lanaset 4 Color Dye Sampler
$13.25
Everything you need to get started with Lanaset dyes:
one 10 gram jar of each of four colors (Sun Yellow, Scarlet, Royal Blue, and Jet Black), plus citric acid crystals, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Buy from
Paradise Fibers
—ADVERTISEMENTS—
Books with Instructions for Using Lanaset Dyes—ADVERTISEMENTS—
Books with Instructions for Using Lanaset DyesMessage: My guild showed me how to dye wool by simmering in white vinegar then putting in a pan of water and sprinkling dye powder on top of the water and covering with foil to form steam which activated the dye. The result was varigated wool. I want to buy the Lanaset dye to do this. Please advise which ones and how much. Thank you.
You can buy Lanaset dyes in some knitting shops, or by mail order from several good dye suppliers. See my blog entry, "Who sells Lanaset dyes?". Paradise Fibers and PRO Chemical & Dye sell a four-color sampler kit which is ideal for beginners.
There will be some instructions that come with the dyes. You can also look at ProChem's instructions for "Rainbow Dyeing using Sabraset/Lanaset Dyes", and at Maiwa's Lanaset-Telana Datasheet [PDF]. Books with instructions for using Lanaset dye include Deb Menz's Color In Spinning, Dagmar Klos's Dyer's Companion, Lynne Vogel's The Twisted Sisters Sock Workbook: Dyeing, Painting, Spinning, Designing, Knitting, and Karren Brito's Shibori: Creating Color And Texture on Silk.
Lanaset dye is the most washfast of all wool dyes, when heat-set for the full recommended period of time, either by immersing the wool and the dye in simmering water for an hour or so, or by steaming the wool over boiling water in a steamer for twenty minutes or so. Unlike other dyes used for wool, it is washfast even in hot water.
(Please help support this web site. Thank you.)
Advertisement

(For silk, wool, angora, mohair & nylon)
Lanaset 4 Color Dye Sampler
$13.25
Everything you need to get started with Lanaset dyes:
one 10 gram jar of each of four colors (Sun Yellow, Scarlet, Royal Blue, and Jet Black), plus citric acid crystals, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Buy from
Paradise Fibers
Wednesday, May 14, 2008
I want to dye my swimsuit. It's 80% polyester and 20% spandex. Would disperse dye work?
Name:
Grace
Message: Hi, I want to dye my swimsuit. It's 80% polyester and 20% spandex. Would disperse dye work? Thank you!
No, you cannot use disperse dye on a swimsuit that contains any spandex at all. Spandex is very heat sensitive. In general, the laundry care instructions tell you to never use water about 105°F (that's 40°C) to wash spandex-containing fabric. This is because the spandex fibers are heat-set. Subjecting them to high heat may cause the shape of your garment to be ruined.
Disperse dyes require high heat to transfer. You must either boil the fabric with the dye for an hour, or apply the dye to paper and then transfer it with a hot iron. In either case, the heat is high enough to almost certainly ruin the swimsuit.
Other types of dye will not work at all. Dyes that work on natural fibers will not stick to polyester. Disperse dyes are the only type of dye that will work at all on polyester, but they cannot be used at temperatures that are low enough to prevent damage to the spandex. This means that polyester/spandex blends are undyeable, in every case.
There is an alternative. You can use an acrylic-based fabric paint, such as Dye-Na-Flow, combined with the additive Jacquard Airfix before use so you don't have to heat-set it. Some fabric paints, such as SetaColor, are reported to become permanent without heat setting or catalyst if they are allowed to dry for a full month. Fabric paint will wear off much more quickly than dye, since it does not penetrate the fibers as deeply, but it is your only alternative for coloring a polyester/spandex blend fabric. It's also more resistant, in many cases, to fading from direct sunlight.
Another option you should consider is buying a new white cotton/spandex swimsuit, which would be very easy to dye in any way you choose with cool water fiber reactive dyes, such as Procion MX dyes. Dharma Trading Company sells a variety of one- and two-piece cotton/spandex bathing suits for very reasonable prices by mail order.
(Please help support this web site. Thank you.)
Message: Hi, I want to dye my swimsuit. It's 80% polyester and 20% spandex. Would disperse dye work? Thank you!
No, you cannot use disperse dye on a swimsuit that contains any spandex at all. Spandex is very heat sensitive. In general, the laundry care instructions tell you to never use water about 105°F (that's 40°C) to wash spandex-containing fabric. This is because the spandex fibers are heat-set. Subjecting them to high heat may cause the shape of your garment to be ruined.
Disperse dyes require high heat to transfer. You must either boil the fabric with the dye for an hour, or apply the dye to paper and then transfer it with a hot iron. In either case, the heat is high enough to almost certainly ruin the swimsuit.
Other types of dye will not work at all. Dyes that work on natural fibers will not stick to polyester. Disperse dyes are the only type of dye that will work at all on polyester, but they cannot be used at temperatures that are low enough to prevent damage to the spandex. This means that polyester/spandex blends are undyeable, in every case.
There is an alternative. You can use an acrylic-based fabric paint, such as Dye-Na-Flow, combined with the additive Jacquard Airfix before use so you don't have to heat-set it. Some fabric paints, such as SetaColor, are reported to become permanent without heat setting or catalyst if they are allowed to dry for a full month. Fabric paint will wear off much more quickly than dye, since it does not penetrate the fibers as deeply, but it is your only alternative for coloring a polyester/spandex blend fabric. It's also more resistant, in many cases, to fading from direct sunlight.
Another option you should consider is buying a new white cotton/spandex swimsuit, which would be very easy to dye in any way you choose with cool water fiber reactive dyes, such as Procion MX dyes. Dharma Trading Company sells a variety of one- and two-piece cotton/spandex bathing suits for very reasonable prices by mail order.
(Please help support this web site. Thank you.)
Tuesday, May 13, 2008
I have bought a viscose "denim coloured" two piece, and I was wondering if there is any treatment that would hold the colour
Name: Mrs
O'Neill
will not work on indigo denim
Message: I have bought a viscose "denim coloured" two piece, and I was wondering if there is any treatment that would hold the colour, as it rubs onto undergarments, and if it is hot and sticky, onto the skin.
I would be very grateful if there was a "quick fix".
No, I'm sorry, there is no quick fix for this particular dye problem.
The dye used in blue denim is called indigo. It can be used to dye any natural fiber, though, not only denim. Indigo is a vat dye, and like all vat dyes is applied in a very different way than other types of dye. Instead of bonding to the fiber, the dye is converted into a water-soluble form and allowed to soak into the fiber, after which it is exposed to air, which converts it to a form which is no longer soluble in water, so the particles of dye become stuck within the fiber.
When dye rubs off of indigo-dyed fabric, it is a manufacturing defect called crocking. The cause is incorrect dye application. Indigo dye should be applied by repeatedly dipping the fabric in the indigo dyebath, allowing the dye to oxidize in between dips. Sometimes the dyer skimps on effort, using fewer dips in an excessively strong dye solution, resulting in loose dye on the surface of the fabric, which easily rubs off.
There is no way to fix the loose surface dye into place when it has been misapplied in this way. Cationic dye fixatives such as Retayne are ineffective for indigo due to its lack of a negative electrical charge.
The best answer is to return the defective garment to the retailer from which you bought it, for a refund. If that is impossible, you might be able to remove enough of the loose dye from the surface of your outfit by washing it repeatedly, perhaps as many as ten times, or more, using the hottest water that the fabric can tolerate. Be very careful in handling viscose rayon when it is wet, because it lacks wet strength and is easily torn.
(Please help support this web site. Thank you.)
—ADVERTISEMENTS—
Retaynewill not work on indigo denim
Message: I have bought a viscose "denim coloured" two piece, and I was wondering if there is any treatment that would hold the colour, as it rubs onto undergarments, and if it is hot and sticky, onto the skin.
I would be very grateful if there was a "quick fix".
No, I'm sorry, there is no quick fix for this particular dye problem.
The dye used in blue denim is called indigo. It can be used to dye any natural fiber, though, not only denim. Indigo is a vat dye, and like all vat dyes is applied in a very different way than other types of dye. Instead of bonding to the fiber, the dye is converted into a water-soluble form and allowed to soak into the fiber, after which it is exposed to air, which converts it to a form which is no longer soluble in water, so the particles of dye become stuck within the fiber.
When dye rubs off of indigo-dyed fabric, it is a manufacturing defect called crocking. The cause is incorrect dye application. Indigo dye should be applied by repeatedly dipping the fabric in the indigo dyebath, allowing the dye to oxidize in between dips. Sometimes the dyer skimps on effort, using fewer dips in an excessively strong dye solution, resulting in loose dye on the surface of the fabric, which easily rubs off.
There is no way to fix the loose surface dye into place when it has been misapplied in this way. Cationic dye fixatives such as Retayne are ineffective for indigo due to its lack of a negative electrical charge.
The best answer is to return the defective garment to the retailer from which you bought it, for a refund. If that is impossible, you might be able to remove enough of the loose dye from the surface of your outfit by washing it repeatedly, perhaps as many as ten times, or more, using the hottest water that the fabric can tolerate. Be very careful in handling viscose rayon when it is wet, because it lacks wet strength and is easily torn.
(Please help support this web site. Thank you.)
Can the shirts stay over the weekend in plastic to remain wet after dyeing (tie dyeing) before rinsing?
Name: Eva
Message: We are doing a group session with students. Can the shirts stay over the weekend in plastic to remain wet after dyeing (tie dyeing) before rinsing?
Yes. The soda ash from the presoak will help to inhibit mold from growing on the shirts over the weekend.
You are using Procion MX dyes with soda ash, or a good tie-dye kit, right? All-purpose dyes, such as Rit, give poor results for tie-dyeing. Be sure to use 100% cotton shirts that are not stain-resistant.
Plastic is not necessary if you use urea to maintain moisture, but it will probably be more convenient for you to store your wet dyed shirts in plastic than to leave them out.
(Please help support this web site. Thank you.)
Message: We are doing a group session with students. Can the shirts stay over the weekend in plastic to remain wet after dyeing (tie dyeing) before rinsing?
Yes. The soda ash from the presoak will help to inhibit mold from growing on the shirts over the weekend.
You are using Procion MX dyes with soda ash, or a good tie-dye kit, right? All-purpose dyes, such as Rit, give poor results for tie-dyeing. Be sure to use 100% cotton shirts that are not stain-resistant.
Plastic is not necessary if you use urea to maintain moisture, but it will probably be more convenient for you to store your wet dyed shirts in plastic than to leave them out.
(Please help support this web site. Thank you.)
Monday, May 12, 2008
how to dye a black t-shirt to give it a more vintage distressed look
Name: Melissa
Message: Hi
I did try doing a search before I emailed you so I hope the question isn't already answered ...
I was wanting to dye a black t-shirt to give it a more vintage distressed look, I'd heard about rock salt, or an antiquing method using RIT dyes but can't find any info about either of these two methods.
One very popular way to give a newly dyed shirt an aged look is to avoid the use of true dye altogether, and use something called pigment dye, instead. In spite of its name, pigment dye does not contain any dyes. What it is is a fabric paint that can be used like a dye.
The pigments in fabric paint do not penetrate the fiber like the molecules of dissolved dye do. Instead, they coat the outside of the fiber. It's been described as the difference between a beet and a radish: a beet is red all the way through, whereas a radish is red on the outside, but white on the inside. This means that the colors in pigment dye are highly susceptible to wear, so they look aged very quickly. It's also difficult to get a perfectly even smooth solid color when coloring whole garments with pigment dyes, which adds to this effect.
There are different sources for pigment dyes. You can use any good high-quality fabric paint that flows like dye, such as Jacquard's Dye-Na-Flow, diluting with the maximum amount of water recommended by the manufacturer. You can use Dharma Pigment Dyes, purchased from Dharma Trading Company in the US. Or, you can use PRO Chemical & Dye's PROfab Pigment Color Concentrates; see their instructions. In Australia, you can mail-order pigment dye concentrates similar to ProChem's from Batik Oetoro. The pigment concentrates would be better for pigment dyeing a number of shirts at once; the Dye-Na-Flow fabric paint or Dharma Pigment Dyes would be better for immersion-coloring a single shirt, or for tie-"dyeing" with pigment dyes (a great solution for the problem of how to tie-dye polyester, by the way). You can mail-order Dye-Na-Flow fabric paint in Australia from The Thread Studio.
In addition to pigment "dyes", there are other possibilities as well. A pretty cool-looking result can be obtained by using a Procion MX black dye in
the dyeing technique known as "low water
immersion". This is the easiest form of dyeing possible. It is much
less trouble than tie-dyeing or
than dyeing a perfectly smooth solid
color. Since all black Procion MX dyes are mixtures of several dye
colors, the different colors separate out on the fabric. Look at the top picture
on this forum posting, "Black Dyes: mixtures and
single-color blacks", to see what it looks like when you use a black
Procion MX dye mixture to do low water immersion dyeing on a white shirt. I
highly recommend this process, if you like the look in the picture. You can
probably obtain a similar result by using Tintex Cool Water Dyes, which are
available in Australia, but avoid Tintex Hot Water Dyes and their North American
equivalent, Tintex Easy Fabric Dye, which are all-purpose
dyes.
in
the dyeing technique known as "low water
immersion". This is the easiest form of dyeing possible. It is much
less trouble than tie-dyeing or
than dyeing a perfectly smooth solid
color. Since all black Procion MX dyes are mixtures of several dye
colors, the different colors separate out on the fabric. Look at the top picture
on this forum posting, "Black Dyes: mixtures and
single-color blacks", to see what it looks like when you use a black
Procion MX dye mixture to do low water immersion dyeing on a white shirt. I
highly recommend this process, if you like the look in the picture. You can
probably obtain a similar result by using Tintex Cool Water Dyes, which are
available in Australia, but avoid Tintex Hot Water Dyes and their North American
equivalent, Tintex Easy Fabric Dye, which are all-purpose
dyes.
I hate to recommend the use of Rit or Tintex all-purpose dye on cotton because of the way it bleeds every single time you wash it. You're supposed to hand-wash any garment you have dyed with Rit dye, separately, using cool water. That is too much trouble for me: I like to throw everything in the washer together, and it works fine for me, simply because I use better dyes. Any clothing dyes with all-purpose dye will ruin other clothing you wash it with, if you are not careful about sorting clothes before washing them, a chore that is entirely unnecessary if you use more washfast dyes, such as Procion MX dyes. Also, I have seen many complaints about getting colors that are not dark enough when dyeing with all-purpose dye. Many people find it disappointing to buy all-purpose dye in black, and then have their clothing turn out not to be black at all after dyeing, but instead an off-gray. As a general rule, it's a good idea to use two to four times as much dye, per shirt, if you are using black dye, as you would for any other color. The fact that all-purpose dye fades quickly might work to your advantage if what you want is an old-looking shirt. However, to my mind, it's not worth the laundry trouble. You can fix Rit dye by after-treating the dyed garments with a mail-order commercial dye fixative, known as Retayne; see "Commercial Dye Fixatives".
A very interesting approach is to begin with a new black t-shirt and discharge-dye it, using diluted bleach. The use of bleach on dark-colored dyes tends to produce oddly antique, ghostly, faded results. Not all black clothing will lose color when treated with bleach; some will stay black no matter what you treat it with. You can dye your own shirts, or (in the US) you can buy black t-shirts from Dharma Trading Company which are guaranteed to react to bleach. I have had good luck in bleaching Hanes and Fruit-of-the-Loom brand black shirts. Be careful when working with chlorine bleach, because it is far more hazardous than any of the dyes we use: be sure to have excellent ventilation so that you do not breathe the fumes (at least have a strong fan in a nearby window, blowing air OUT the window, or, preferably, wear a good respirator with acid-gas cartridges), and wear good strong gloves, not the very thin disposable gloves that oven spring a leak.
I have read about washing denim with rock salt to distress the fabric, by adding three cups of rock salt to a small load of wash. I do not recommend this, because if the rock salt stays undissolved long enough to abrade your fabric like little pebbles, it is also going to be damaging the lining of your washing machine; if the rock salt dissolves too quickly to damage the washer, then it is not going to be effective in distressing your fabric. If you want to physically damage your fabric slightly, to give it a truly aged look, then I recommend you do so by using sandpaper to rub the garment directly by hand, without risking major damage to your washing machine. I dye clothing in my washing machine all the time without damaging the machine, but physical abrasives are sure to be much more damaging than dye.
(Please help support this web site. Thank you.)
Message: Hi
I did try doing a search before I emailed you so I hope the question isn't already answered ...
I was wanting to dye a black t-shirt to give it a more vintage distressed look, I'd heard about rock salt, or an antiquing method using RIT dyes but can't find any info about either of these two methods.
One very popular way to give a newly dyed shirt an aged look is to avoid the use of true dye altogether, and use something called pigment dye, instead. In spite of its name, pigment dye does not contain any dyes. What it is is a fabric paint that can be used like a dye.
The pigments in fabric paint do not penetrate the fiber like the molecules of dissolved dye do. Instead, they coat the outside of the fiber. It's been described as the difference between a beet and a radish: a beet is red all the way through, whereas a radish is red on the outside, but white on the inside. This means that the colors in pigment dye are highly susceptible to wear, so they look aged very quickly. It's also difficult to get a perfectly even smooth solid color when coloring whole garments with pigment dyes, which adds to this effect.
There are different sources for pigment dyes. You can use any good high-quality fabric paint that flows like dye, such as Jacquard's Dye-Na-Flow, diluting with the maximum amount of water recommended by the manufacturer. You can use Dharma Pigment Dyes, purchased from Dharma Trading Company in the US. Or, you can use PRO Chemical & Dye's PROfab Pigment Color Concentrates; see their instructions. In Australia, you can mail-order pigment dye concentrates similar to ProChem's from Batik Oetoro. The pigment concentrates would be better for pigment dyeing a number of shirts at once; the Dye-Na-Flow fabric paint or Dharma Pigment Dyes would be better for immersion-coloring a single shirt, or for tie-"dyeing" with pigment dyes (a great solution for the problem of how to tie-dye polyester, by the way). You can mail-order Dye-Na-Flow fabric paint in Australia from The Thread Studio.
In addition to pigment "dyes", there are other possibilities as well. A pretty cool-looking result can be obtained by using a Procion MX black dye
 in
the dyeing technique known as "low water
immersion". This is the easiest form of dyeing possible. It is much
less trouble than tie-dyeing or
than dyeing a perfectly smooth solid
color. Since all black Procion MX dyes are mixtures of several dye
colors, the different colors separate out on the fabric. Look at the top picture
on this forum posting, "Black Dyes: mixtures and
single-color blacks", to see what it looks like when you use a black
Procion MX dye mixture to do low water immersion dyeing on a white shirt. I
highly recommend this process, if you like the look in the picture. You can
probably obtain a similar result by using Tintex Cool Water Dyes, which are
available in Australia, but avoid Tintex Hot Water Dyes and their North American
equivalent, Tintex Easy Fabric Dye, which are all-purpose
dyes.
in
the dyeing technique known as "low water
immersion". This is the easiest form of dyeing possible. It is much
less trouble than tie-dyeing or
than dyeing a perfectly smooth solid
color. Since all black Procion MX dyes are mixtures of several dye
colors, the different colors separate out on the fabric. Look at the top picture
on this forum posting, "Black Dyes: mixtures and
single-color blacks", to see what it looks like when you use a black
Procion MX dye mixture to do low water immersion dyeing on a white shirt. I
highly recommend this process, if you like the look in the picture. You can
probably obtain a similar result by using Tintex Cool Water Dyes, which are
available in Australia, but avoid Tintex Hot Water Dyes and their North American
equivalent, Tintex Easy Fabric Dye, which are all-purpose
dyes.I hate to recommend the use of Rit or Tintex all-purpose dye on cotton because of the way it bleeds every single time you wash it. You're supposed to hand-wash any garment you have dyed with Rit dye, separately, using cool water. That is too much trouble for me: I like to throw everything in the washer together, and it works fine for me, simply because I use better dyes. Any clothing dyes with all-purpose dye will ruin other clothing you wash it with, if you are not careful about sorting clothes before washing them, a chore that is entirely unnecessary if you use more washfast dyes, such as Procion MX dyes. Also, I have seen many complaints about getting colors that are not dark enough when dyeing with all-purpose dye. Many people find it disappointing to buy all-purpose dye in black, and then have their clothing turn out not to be black at all after dyeing, but instead an off-gray. As a general rule, it's a good idea to use two to four times as much dye, per shirt, if you are using black dye, as you would for any other color. The fact that all-purpose dye fades quickly might work to your advantage if what you want is an old-looking shirt. However, to my mind, it's not worth the laundry trouble. You can fix Rit dye by after-treating the dyed garments with a mail-order commercial dye fixative, known as Retayne; see "Commercial Dye Fixatives".
A very interesting approach is to begin with a new black t-shirt and discharge-dye it, using diluted bleach. The use of bleach on dark-colored dyes tends to produce oddly antique, ghostly, faded results. Not all black clothing will lose color when treated with bleach; some will stay black no matter what you treat it with. You can dye your own shirts, or (in the US) you can buy black t-shirts from Dharma Trading Company which are guaranteed to react to bleach. I have had good luck in bleaching Hanes and Fruit-of-the-Loom brand black shirts. Be careful when working with chlorine bleach, because it is far more hazardous than any of the dyes we use: be sure to have excellent ventilation so that you do not breathe the fumes (at least have a strong fan in a nearby window, blowing air OUT the window, or, preferably, wear a good respirator with acid-gas cartridges), and wear good strong gloves, not the very thin disposable gloves that oven spring a leak.
I have read about washing denim with rock salt to distress the fabric, by adding three cups of rock salt to a small load of wash. I do not recommend this, because if the rock salt stays undissolved long enough to abrade your fabric like little pebbles, it is also going to be damaging the lining of your washing machine; if the rock salt dissolves too quickly to damage the washer, then it is not going to be effective in distressing your fabric. If you want to physically damage your fabric slightly, to give it a truly aged look, then I recommend you do so by using sandpaper to rub the garment directly by hand, without risking major damage to your washing machine. I dye clothing in my washing machine all the time without damaging the machine, but physical abrasives are sure to be much more damaging than dye.
(Please help support this web site. Thank you.)
Sunday, May 11, 2008
Do you take special orders? I'd like a shirt made, but not sure about finding someone to do it.
Name:
Desiree
Message: Do you take special orders? I'd like a shirt made, but not sure about finding someone to do it. Please let me know. Thank you :)
I don't, but I know people who do. Look on my website on the page, "Find a Custom Dyer". Scroll down to the second section, where there are listed many custom tie-dyers, such as Wildflower Tie Dye.
You may also post on the Dye Forum describing what you'd like and asking for a dyer to make it for you.
(Please help support this web site. Thank you.)
Message: Do you take special orders? I'd like a shirt made, but not sure about finding someone to do it. Please let me know. Thank you :)
I don't, but I know people who do. Look on my website on the page, "Find a Custom Dyer". Scroll down to the second section, where there are listed many custom tie-dyers, such as Wildflower Tie Dye.
You may also post on the Dye Forum describing what you'd like and asking for a dyer to make it for you.
(Please help support this web site. Thank you.)
Saturday, May 10, 2008
I have red cotton slipcovers that have faded from sun and washing
Name:
Joan
Message: I have red cotton slipcovers that have faded from sun and washing.
What kind of dye should I use to perk up the red, and can I use my washing machine to dye these slip covers? They come off in sections: arms, cushions, skirts, etc.
The easiest way to dye your cotton slipcovers will be in the washing machine, using Procion MX or another good type of fiber reactive dye.
Will they all fit into one washing machine load? If not, you will have to go to some effort to make sure that the conditions are exactly the same in each of the loads that you dye, so that the color will come out the same. If you dye a large amount of fabric in one washing machine load, and a lesser amount in another washing machine load, the two or three batches will come out different colors.
If you will have to divide the slipcovers into two or more loads for dyeing, you must weigh them, and make sure to have two or three loads that contain exactly the same weight of fabric. Be careful to fill the washing machine to the same level, so as to use the same amount of water, and make a careful note of the time at which you put in each of your dyebath ingredients (dye, salt, soda ash, fabric), so as to run each load for exactly the same amount of time with the dye. Measure your dye carefully, as well as your salt and your soda ash, to be sure to use the same amount of each thing.
If you live in Europe or Australia, use Dylon Machine dye, which contains a good fiber reactive dye known as Drimarene K dye. This dye is not available in North America, but you will get excellent results by following a good recipe for washing machine dyeing with Procion MX dye.
You should be aware that, because dye is transparent, areas that are heavily faded now will still be lighter in color than the other areas, after you dye them. Very dark or intense dye colors will help to cover up differences. If you use a dye that is the same color as the original dye, you may find that using a larger amount of dye, two to three times as much as the recipe requires, will make a red that is intense enough to help cover up any differences.
For more information, see "How can I dye clothing or fabric in the washing machine?".
(Please help support this web site. Thank you.)
Message: I have red cotton slipcovers that have faded from sun and washing.
What kind of dye should I use to perk up the red, and can I use my washing machine to dye these slip covers? They come off in sections: arms, cushions, skirts, etc.
The easiest way to dye your cotton slipcovers will be in the washing machine, using Procion MX or another good type of fiber reactive dye.
Will they all fit into one washing machine load? If not, you will have to go to some effort to make sure that the conditions are exactly the same in each of the loads that you dye, so that the color will come out the same. If you dye a large amount of fabric in one washing machine load, and a lesser amount in another washing machine load, the two or three batches will come out different colors.
If you will have to divide the slipcovers into two or more loads for dyeing, you must weigh them, and make sure to have two or three loads that contain exactly the same weight of fabric. Be careful to fill the washing machine to the same level, so as to use the same amount of water, and make a careful note of the time at which you put in each of your dyebath ingredients (dye, salt, soda ash, fabric), so as to run each load for exactly the same amount of time with the dye. Measure your dye carefully, as well as your salt and your soda ash, to be sure to use the same amount of each thing.
If you live in Europe or Australia, use Dylon Machine dye, which contains a good fiber reactive dye known as Drimarene K dye. This dye is not available in North America, but you will get excellent results by following a good recipe for washing machine dyeing with Procion MX dye.
You should be aware that, because dye is transparent, areas that are heavily faded now will still be lighter in color than the other areas, after you dye them. Very dark or intense dye colors will help to cover up differences. If you use a dye that is the same color as the original dye, you may find that using a larger amount of dye, two to three times as much as the recipe requires, will make a red that is intense enough to help cover up any differences.
For more information, see "How can I dye clothing or fabric in the washing machine?".
(Please help support this web site. Thank you.)
Friday, May 09, 2008
Can I dye a table cloth (100% polyester) that is red to a Navy Blue?
Name:
juanna
Message: Can I dye a table cloth (100% polyester) that is red to a Navy Blue?
How Would I do that?
No, I don't think you want to do that. While it is possible to dye polyester by boiling it for an hour with a special polyester dye called disperse dye, you would need a large non-aluminum cooking pot, large enough for your tablecloth to move in freely while submerged; this cooking pot should never again be used for food, after you've used it to dye fabric.
Unless you are going to be dyeing a large number of polyester items, it will not be worthwhile to invest in such an enormous cooking pot. A new tablecloth would cost a lot less!
The best thing for you to do would be to buy a new navy blue tablecloth, or buy a dyeable white PFD cotton tablecloth. Dharma Trading Company sells cotton tablecloths and placemats that are suitable for dyeing. Be sure to buy only items that have been sewn with cotton thread, as otherwise the thread will remain the origional color you dyeing. You can easily dye cotton using Procion MX dye, following the "How to Dye With Fiber Reactive Dyes" recipe. Unfortunately, you cannot dye polyester with fiber reactive dye.
For more information about dyeing polyester, see "Dyeing Polyester with Disperse Dyes".
(Please help support this web site. Thank you.)
Message: Can I dye a table cloth (100% polyester) that is red to a Navy Blue?
How Would I do that?
No, I don't think you want to do that. While it is possible to dye polyester by boiling it for an hour with a special polyester dye called disperse dye, you would need a large non-aluminum cooking pot, large enough for your tablecloth to move in freely while submerged; this cooking pot should never again be used for food, after you've used it to dye fabric.
Unless you are going to be dyeing a large number of polyester items, it will not be worthwhile to invest in such an enormous cooking pot. A new tablecloth would cost a lot less!
The best thing for you to do would be to buy a new navy blue tablecloth, or buy a dyeable white PFD cotton tablecloth. Dharma Trading Company sells cotton tablecloths and placemats that are suitable for dyeing. Be sure to buy only items that have been sewn with cotton thread, as otherwise the thread will remain the origional color you dyeing. You can easily dye cotton using Procion MX dye, following the "How to Dye With Fiber Reactive Dyes" recipe. Unfortunately, you cannot dye polyester with fiber reactive dye.
For more information about dyeing polyester, see "Dyeing Polyester with Disperse Dyes".
(Please help support this web site. Thank you.)
Thursday, May 08, 2008
How can I get a solid even color when dyeing nylon gloves?
Name:
Barbaran
Message: Good Day
I am wishing to dye nylon gloves. I am using Jacquard Acid dyes. The colour I wish to achieve is lilac and I am mixing periwinkle 616 with touch of hot fuchsia 620 which gives me the perfect colour on a test swatch. I pre washed the gloves, however when I immerse the gloves into the dye mix the dye splits and parts the gloves are blue while the other parts are pinkish. If I use only one colur I don't experience a problem, however this time I need to mix two colours to get the match I require. Can you tell me why the dye is splitting. These gloves are required for a wedding on the 24th May so I don’t have much time to get it right. Can you help?
Acid dyes are a good choice for dyeing nylon fabric. Nylon should dye well if it is free of surface finishes that can impair dye penetration.
Unfortunately, finishes are common on nylon and can interfere badly with dyeing. The best nylon is labeled "PFP" for "Prepared For Printing" or "PFD" for "Prepared For Dyeing", but that's unlikely to be available in gloves. Some finishes will be removed by rigorous scouring (which is washing in very hot water with detergent plus washing soda), while others will not be fully removed no matter what you do. Permanent-press finishes and stain-resistant finishes are the worst, but there are also other finishes that may not be indicated on the package label.
Increasing the smoothness of a solid dye color is helped by several factors. One is using a large amount of water. If you were dyeing only one pair of gloves, then a four-liter pot should probably allow plenty of room for the fabric to move freely. Stirring more frequently will help to encourage a solid even color.
Do you paste up your dyes? To dissolve dyes, first mix them with a few drops of water, adding more water as necessary to make a thick smooth paste, Then gradually add more water to dissolve thoroughly. Finally, strain the dye solutions through a coffee filter or the toe of sheer nylon stocking, to remove any undissolved clumps.
The fabric needs to be thoroughly wetted before dyeing. Wet out the fabric by soaking it in warm water with a single tiny drop of Synthrapol or liquid hand dishwashing detergent. Soaking overnight will be most effective, but at least soak for half an hour.
Acid dyes are supposed to be used with an acid, such as vinegar or citric acid. It appears that you added no acid at all to your dyebath. Nylon has a much greater affinity to dye at a low (acid) pH. Not adding any acid could be a big error; adding acid might make a big difference in your results. Here are Jacquard's instructions for dyeing with their acid dyes, which require the use of distilled white vinegar, which is 5% acetic acid:
[from http://www.jacquardproducts.com/products/dyes/aciddye/instructions2.php]
Message: Good Day
I am wishing to dye nylon gloves. I am using Jacquard Acid dyes. The colour I wish to achieve is lilac and I am mixing periwinkle 616 with touch of hot fuchsia 620 which gives me the perfect colour on a test swatch. I pre washed the gloves, however when I immerse the gloves into the dye mix the dye splits and parts the gloves are blue while the other parts are pinkish. If I use only one colur I don't experience a problem, however this time I need to mix two colours to get the match I require. Can you tell me why the dye is splitting. These gloves are required for a wedding on the 24th May so I don’t have much time to get it right. Can you help?
Acid dyes are a good choice for dyeing nylon fabric. Nylon should dye well if it is free of surface finishes that can impair dye penetration.
Unfortunately, finishes are common on nylon and can interfere badly with dyeing. The best nylon is labeled "PFP" for "Prepared For Printing" or "PFD" for "Prepared For Dyeing", but that's unlikely to be available in gloves. Some finishes will be removed by rigorous scouring (which is washing in very hot water with detergent plus washing soda), while others will not be fully removed no matter what you do. Permanent-press finishes and stain-resistant finishes are the worst, but there are also other finishes that may not be indicated on the package label.
Increasing the smoothness of a solid dye color is helped by several factors. One is using a large amount of water. If you were dyeing only one pair of gloves, then a four-liter pot should probably allow plenty of room for the fabric to move freely. Stirring more frequently will help to encourage a solid even color.
Do you paste up your dyes? To dissolve dyes, first mix them with a few drops of water, adding more water as necessary to make a thick smooth paste, Then gradually add more water to dissolve thoroughly. Finally, strain the dye solutions through a coffee filter or the toe of sheer nylon stocking, to remove any undissolved clumps.
The fabric needs to be thoroughly wetted before dyeing. Wet out the fabric by soaking it in warm water with a single tiny drop of Synthrapol or liquid hand dishwashing detergent. Soaking overnight will be most effective, but at least soak for half an hour.
Acid dyes are supposed to be used with an acid, such as vinegar or citric acid. It appears that you added no acid at all to your dyebath. Nylon has a much greater affinity to dye at a low (acid) pH. Not adding any acid could be a big error; adding acid might make a big difference in your results. Here are Jacquard's instructions for dyeing with their acid dyes, which require the use of distilled white vinegar, which is 5% acetic acid:
- Fill a stainless steel or enamel pot with just enough hot or warm water for the fabric to swim freely, turn on the heat.
- Add the dye powder to the pot and stir. Normally, in this procedure you would add 2 to 4% of the dry weight of the fabric in dye powder. For example, if you are dyeing 1 pound [454 grams] of fabric, use 1/3 to 2/3 of an ounce of dye[10 to 20 grams].
- Add the fabric that has been thoroughly wetted to the dyepot.
- Raise the temperature to 185 to 200°F, just below boiling. [That's 85°C to 93°C.] Stir frequently.
- Add one-quarter cup [60 ml] of vinegar per pound [454 g] of fabric. Try not to pour directly onto the fabric.
- Maintain temperature and stir frequently for thirty minutes. Wash in Synthrapol and warm water. Note: If you are dyeing wool, a gradual heating and gradual cooling of the dyebath is important so as not to shock and felt the wool.
To salvage the unevenly dyed gloves, try soaking in boiling water. Some acid dyes will come out when treated this way. If this does not work, and the gloves are ruined anyway by the uneven color, you may try a sulfur-based dye remover, such as Rit Color Remover, or Dylon Run Away. Do not use chlorine (hypochlorite) bleach, because it destroys nylon.
Periwinkle 615 is a premixed dye, containing two or more other dye colors in it, so I don't know which dyes are in it. Hot Fuchsia 620 is acid red 52, a fluorescent dye also known as Rhodamine B. Rhodamine B is also among the dyes ProChem in the US sells in their Washfast Acid line of dyes. In their instructions they say to wet out the fiber by measuring one-half teaspoon (2.5 ml) Synthrapol in 2.5 gallons (10 liters) of warm 110°F (44°C) water, for each pound (454 gm) of fiber, and soak for at least 30 minutes. Take a look at their instructions. They say to use eleven tablespoons of white vinegar, which is 165 ml, for every 10 liters of water, and they say to include a teaspoon of Synthrapol detergent in the dyebath. The recipe is mostly pretty similar to the Jacquard recipe. If you do not have Synthrapol, use liquid hand dishwashing detergent. This should help with dye penetration and evenness.
(Please help support this web site. Thank you.)
Wednesday, May 07, 2008
How can I make dyes out of just the general reagents available in an ordinary chemistry lab?
Name: Arya
Message: I am suppose to make a project for chemistry in which I am supposed to make dye out of general reagents available in an ordinary lab. Could you help?
I remember a series of labs in my advanced chemistry class in high school in which, if I recall correctly, we used toluene to make nitrobenzene, which we then used to make aniline. The final step was to use our products to make dyes, presumably azo dyes. It was great fun. In my case, I selected the blue dye option, but made some slight error and ended up with green. We mordanted cotton shirts with alum and then dyed them with the dyes we had made, but, as none of us knew anything about dyeing at the time, all of the dyes washed out the first time they were laundered. I remember that just a tiny amount of color remained in my shirt, enough to make it look dirty, but not to give it any real color.
A very old chemistry lab manual, William Simon's 1898 Manual of Chemistry, A Guide to Lectures and Laboratory Work for Beginners in Chemistry, on page 545 says that aniline and toluidine, when "treated with oxidizing agents such as arsenic oxide, hypochlorites [such as household bleach], chromic or nitric acid, etc., various substances are oobtained which are either themselves distinguished by beautiful colors or may be converted into numerous derivatives showing all the various shades of red, blue, violet, green, etc." You can read this book online in Google Books.
Your library should be able to get you the following article via interlibrary loan, if they don't subscribe to the Journal of Chemical Education: "Synthesis of Triarylmethane and Xanthene Dyes Using Electrophilic Aromatic Substitution Reactions" (James V. McCullagh, and Kelly A.Daggett, J. Chem. Educ. 2007, 84: 1799). Here is the abstract:
An online laboratory guide for making dye in a college organic chemistry lab can be found in the Online Chemistry Lab Turtorials at Washington University, in a chapter entitled "Aromatic Chemistry: Synthesis of o-Nitroaniline and p-Nitroaniline via a Multi-Step Sequence":
Another lab experiment is detailed in "Diazonium Coupling Reaction of p-Nitrobenzenediazonium sulfate and N,N -Dimethylaniline: Synthesis of p-(4-nitrobenzeneazo)-N,N-dimethylaniline ", or try "Multi-step Synthesis: Preparation of Organic Dyes".
You can view a video of a lab in which naphthol dyes are synthesized. Here are links to the description of the experiment and to the video.
For methods of synthesizing Procion MX (dichlorotriazine) dyes, see David R. Waring and Geoffrey Hallas's 1990 book, "The Chemistry and Application of Dyes (Topics in Applied Chemistry)". At $192, it is too expensive for a student to buy, but you may be able to find it at the public library or in the library of your educational institution. There are also recipes for the synthesis of dichlorotriazine dyes in the appendix of Victor B. Ivanov's Reactive Dyes in Biology.
With all of these possibilities, I think you should not have any trouble in selecting a suitable method for synthesizing dye out of the reagents available in an ordinary lab.
(Please help support this web site. Thank you.)
Message: I am suppose to make a project for chemistry in which I am supposed to make dye out of general reagents available in an ordinary lab. Could you help?
I remember a series of labs in my advanced chemistry class in high school in which, if I recall correctly, we used toluene to make nitrobenzene, which we then used to make aniline. The final step was to use our products to make dyes, presumably azo dyes. It was great fun. In my case, I selected the blue dye option, but made some slight error and ended up with green. We mordanted cotton shirts with alum and then dyed them with the dyes we had made, but, as none of us knew anything about dyeing at the time, all of the dyes washed out the first time they were laundered. I remember that just a tiny amount of color remained in my shirt, enough to make it look dirty, but not to give it any real color.
A very old chemistry lab manual, William Simon's 1898 Manual of Chemistry, A Guide to Lectures and Laboratory Work for Beginners in Chemistry, on page 545 says that aniline and toluidine, when "treated with oxidizing agents such as arsenic oxide, hypochlorites [such as household bleach], chromic or nitric acid, etc., various substances are oobtained which are either themselves distinguished by beautiful colors or may be converted into numerous derivatives showing all the various shades of red, blue, violet, green, etc." You can read this book online in Google Books.
Your library should be able to get you the following article via interlibrary loan, if they don't subscribe to the Journal of Chemical Education: "Synthesis of Triarylmethane and Xanthene Dyes Using Electrophilic Aromatic Substitution Reactions" (James V. McCullagh, and Kelly A.Daggett, J. Chem. Educ. 2007, 84: 1799). Here is the abstract:
"The synthesis of dyes has long been a popular topic in organic chemistry laboratory experiments because it allows students to see first hand that reactions learned in class can be used to make compounds with useful applications. In this experiment electrophilic aromatic substitution reactions are used to synthesize several triarylmethane and xanthene dyes (fluorescein, erythrosin B, thymolphthalein, and rhodamine B). These dyes can be readily synthesized using equipment found in most common second-year organic labs. Hydroscopic Lewis acids, which are often troublesome to use in a typical lab setup, are avoided. The dyes synthesized in this experiment can each be completed in one four-hour laboratory session. Approximately 30 minutes in a subsequent period will be required for UV–vis analysis, and the student samples give UV–vis spectra that match commercially available dye samples."
An online laboratory guide for making dye in a college organic chemistry lab can be found in the Online Chemistry Lab Turtorials at Washington University, in a chapter entitled "Aromatic Chemistry: Synthesis of o-Nitroaniline and p-Nitroaniline via a Multi-Step Sequence":
"The purpose of this experiment is to prepare ortho- and para-nitroaniline from nitrobenzene. Para-nitroaniline is used in the synthesis of the dye para red, also known as American flag red. You have the option to use your product to synthesize this dye when you have finished the experiment."
Another lab experiment is detailed in "Diazonium Coupling Reaction of p-Nitrobenzenediazonium sulfate and N,N -Dimethylaniline: Synthesis of p-(4-nitrobenzeneazo)-N,N-dimethylaniline ", or try "Multi-step Synthesis: Preparation of Organic Dyes".
You can view a video of a lab in which naphthol dyes are synthesized. Here are links to the description of the experiment and to the video.
For methods of synthesizing Procion MX (dichlorotriazine) dyes, see David R. Waring and Geoffrey Hallas's 1990 book, "The Chemistry and Application of Dyes (Topics in Applied Chemistry)". At $192, it is too expensive for a student to buy, but you may be able to find it at the public library or in the library of your educational institution. There are also recipes for the synthesis of dichlorotriazine dyes in the appendix of Victor B. Ivanov's Reactive Dyes in Biology.
With all of these possibilities, I think you should not have any trouble in selecting a suitable method for synthesizing dye out of the reagents available in an ordinary lab.
(Please help support this web site. Thank you.)
Tuesday, May 06, 2008
What is the dye used for clothing typically made from?
What is the dye used for clothing typically made
from?
Almost all dyes typically used for clothing, especially commercially-made clothing, are synthetic, not made from natural sources. They are made from chemicals that ultimately derive from either coal or petroleum.
Indigo, a very popular dye used for blue denim, can be derived from plants such as the indigo plant or woad—there are some fifty species of plants around the world that produce this popular dye—but in actual practice, these days, it is produced synthetically. There are several different processes by which synthetic indigo may be made; in the currently most used industrial method, by which the vast majority of indigo is now made, it is manufactured from indoxyl, which is produced by the fusion of sodium phenylglycinate in a mixture of caustic soda and sodamide. The sodium phenylglycinate used in this reaction can be made from aniline; aniline, in turn, is prepared commercially by the catalytic hydrogenation of nitrobenzene or by the action of ammonia on chlorobenzene. The nitrobenzene is synthesized from benzene, which is obtained from coal or petroleum. So, ultimately, indigo is made from coal or petroleum. Plants are not used because synthesizing dye from fossil fuels is less expensive.
Synthetic dyes in general are often called "aniline dyes", because they were, at first made from aniline, but most dyes are no longer made from aniline. They are, however, synthesized from other chemicals derived from coal or petroleum, so the ultimate source is the same. Most commercially dyed cotton clothing is dyed with sulfur dyes, direct dyes, or reactive dyes, while most wool and nylon clothing is dyed with acid dyes. Polyester and acetate are dyed with disperse dyes, and acrylic and modacrylic are usually dyed with basic dyes.
For many years, direct dye was manufactured from benzidine or o-dianisidine, two chemicals known to be significantly carcinogenic. Unfortunately, the dyes metabolize back to these chemicals in the body, so their use is dangerous. Benzidine-containing dyes have since been largely phased out in the US, but were widely available in the form of packets of all-purpose dye, sold in grocery stores and pharmacies, though the 1970s. Dyes based on o-dianisidine can still be found in some consumer products, such as a "Tie Dye Color Cords" kit made by Consolidated Thread Mills, intended for use by children. Dyes which are not based on benzidine or o-dianisidine are much safer and more suitable for use by hobbyists or children.
Sources and further reading:
• How Indigo Is Made
• Health Hazard Alert-- Benzidine-, o-Tolidine-, and o-Dianisidine- Based Dyes
• Dyes and Dyeing Glossary: A Glossary of Terms for Materials and Processes in Textile Dyeing for Artists
• Aniline (Encyclopaedia Britannica)
• Dyes (Encyclopaedia Britannica)
• The Chemistry and Application of Dyes, edited by David R. Waring and Geoffrey Hallas
(Please help support this web site. Thank you.)
Almost all dyes typically used for clothing, especially commercially-made clothing, are synthetic, not made from natural sources. They are made from chemicals that ultimately derive from either coal or petroleum.
Indigo, a very popular dye used for blue denim, can be derived from plants such as the indigo plant or woad—there are some fifty species of plants around the world that produce this popular dye—but in actual practice, these days, it is produced synthetically. There are several different processes by which synthetic indigo may be made; in the currently most used industrial method, by which the vast majority of indigo is now made, it is manufactured from indoxyl, which is produced by the fusion of sodium phenylglycinate in a mixture of caustic soda and sodamide. The sodium phenylglycinate used in this reaction can be made from aniline; aniline, in turn, is prepared commercially by the catalytic hydrogenation of nitrobenzene or by the action of ammonia on chlorobenzene. The nitrobenzene is synthesized from benzene, which is obtained from coal or petroleum. So, ultimately, indigo is made from coal or petroleum. Plants are not used because synthesizing dye from fossil fuels is less expensive.
Synthetic dyes in general are often called "aniline dyes", because they were, at first made from aniline, but most dyes are no longer made from aniline. They are, however, synthesized from other chemicals derived from coal or petroleum, so the ultimate source is the same. Most commercially dyed cotton clothing is dyed with sulfur dyes, direct dyes, or reactive dyes, while most wool and nylon clothing is dyed with acid dyes. Polyester and acetate are dyed with disperse dyes, and acrylic and modacrylic are usually dyed with basic dyes.
For many years, direct dye was manufactured from benzidine or o-dianisidine, two chemicals known to be significantly carcinogenic. Unfortunately, the dyes metabolize back to these chemicals in the body, so their use is dangerous. Benzidine-containing dyes have since been largely phased out in the US, but were widely available in the form of packets of all-purpose dye, sold in grocery stores and pharmacies, though the 1970s. Dyes based on o-dianisidine can still be found in some consumer products, such as a "Tie Dye Color Cords" kit made by Consolidated Thread Mills, intended for use by children. Dyes which are not based on benzidine or o-dianisidine are much safer and more suitable for use by hobbyists or children.
Sources and further reading:
• How Indigo Is Made
• Health Hazard Alert-- Benzidine-, o-Tolidine-, and o-Dianisidine- Based Dyes
• Dyes and Dyeing Glossary: A Glossary of Terms for Materials and Processes in Textile Dyeing for Artists
• Aniline (Encyclopaedia Britannica)
• Dyes (Encyclopaedia Britannica)
• The Chemistry and Application of Dyes, edited by David R. Waring and Geoffrey Hallas
(Please help support this web site. Thank you.)
Monday, May 05, 2008
Can one set the colors for tie dying with vinegar when the project is complete rather than buying the sodium carbonate and soaking the fabric before dying?
Name:
Beth
Message: Can one set the colors for tie dying with vinegar when the project is complete rather than buying the sodium carbonate and soaking the fabric before dying? Thanks!
No. Vinegar is useless, if you are dyeing cotton. It will do nothing to set the dye.
If you are using fiber reactive dye to tie-dye cotton, or any other cellulose fiber such as linen or rayon, you MUST use a high-pH chemical such as soda ash (sodium carbonate) to set the dye. You cannot use vinegar.
However, if you are using an all-purpose dye such as Rit, neither vinegar nor soda ash will set the dye. The only way to set Rit dye on cotton, so that it does not bleed badly in the laundry, is to buy a commercial dye fixative such as Retayne. Your local shops are unlikely to carry Retayne, so you will have to buy it buy mail-order. Even with the use of Retayne, all-purpose dye is inferior to fiber reactive dyes, such as Procion MX dye.
If you buy a good tie-dye kit, the soda ash will be included in the kit, so you will not need to buy it at all. Good tie dye kits are made by Jacquard Products, Rainbow Rock, Tulip, Dylon, and Dritz. The Jacquard Products Tie-Dye Kits contain soda ash to use as a separate pre-soak, whereas the Tulip Tie-Dye Kits contain soda ash mixed in with the dye powder. Avoid tie-dye kits that contain hot-water dye, such as the Rit Tie Dye Kit or the Magic String Tie-Dye Kit, because the colors are disappointing, they fade quickly, and they bleed forever in the laundry.
It is easy to buy sodium carbonate. Hardware stores and swimming pool supply stores sell it as "pH Up". Check the fine print on the label to make sure that it is sodium carbonate, not sodium BIcarbonate. You can also substitute washing soda from the grocery store, if it is free of additives such as dyes and perfumes. See "What is soda ash, and what's it for in dyeing?".
Soda ash can be used to set Procion MX dye (never Rit dye) by adding it to the fabric either as a presoak before adding the dye, by mixing it with the dye, or by applying the soda ash afterwards. After-fixing is not very suitable for tie-dyeing, however, because the dye will run and bleed in the soda ash before it completes setting. It is better to use the soda ash first, before the dye.
If you have all-purpose dye and are intending to use it for tie-dyeing, please change your mind. Return the unopened packages of all-purpose dye to the store, or throw it away. It can only give poor results in tie-dyeing. You will get vastly better results if you use cool water fiber reactive dyes, made by Jacquard Products, Rainbow Rock, Tulip, Dylon, or Dritz. Rit is a hot water dye and should be applied only in scalding hot water, not at room temperature; see the instructions under "How can I tie dye with RIT dye?". If you've only used Rit dye in the past, you will be amazed at how much richer and brighter are the colors that Procion MX dyes produce, and how much longer they last in the laundry.
Vinegar is useful only when you are dyeing wool or silk with acid dyes. Wool and silk are chemically very different from cotton, so they can be used with vinegar, although cotton cannot. You can use Rit dye to tie-dye wool, silk, or nylon, if you presoak the fabric in vinegar, and follow the dyeing by wrapping the still-wet dyed fabric up in plastic wrap and steaming it in a covered pot over boiling water for at least half an hour, much as you would steam vegetables. Heat-setting is required if you are using Rit dye. If you use a cooking pot to immersion dye with Rit dye, do not reuse the pot for cooking food, because Rit dye is not safe for use on food preparation utensils and equipment.
(Please help support this web site. Thank you.)
Message: Can one set the colors for tie dying with vinegar when the project is complete rather than buying the sodium carbonate and soaking the fabric before dying? Thanks!
No. Vinegar is useless, if you are dyeing cotton. It will do nothing to set the dye.
If you are using fiber reactive dye to tie-dye cotton, or any other cellulose fiber such as linen or rayon, you MUST use a high-pH chemical such as soda ash (sodium carbonate) to set the dye. You cannot use vinegar.
However, if you are using an all-purpose dye such as Rit, neither vinegar nor soda ash will set the dye. The only way to set Rit dye on cotton, so that it does not bleed badly in the laundry, is to buy a commercial dye fixative such as Retayne. Your local shops are unlikely to carry Retayne, so you will have to buy it buy mail-order. Even with the use of Retayne, all-purpose dye is inferior to fiber reactive dyes, such as Procion MX dye.
If you buy a good tie-dye kit, the soda ash will be included in the kit, so you will not need to buy it at all. Good tie dye kits are made by Jacquard Products, Rainbow Rock, Tulip, Dylon, and Dritz. The Jacquard Products Tie-Dye Kits contain soda ash to use as a separate pre-soak, whereas the Tulip Tie-Dye Kits contain soda ash mixed in with the dye powder. Avoid tie-dye kits that contain hot-water dye, such as the Rit Tie Dye Kit or the Magic String Tie-Dye Kit, because the colors are disappointing, they fade quickly, and they bleed forever in the laundry.
It is easy to buy sodium carbonate. Hardware stores and swimming pool supply stores sell it as "pH Up". Check the fine print on the label to make sure that it is sodium carbonate, not sodium BIcarbonate. You can also substitute washing soda from the grocery store, if it is free of additives such as dyes and perfumes. See "What is soda ash, and what's it for in dyeing?".
Soda ash can be used to set Procion MX dye (never Rit dye) by adding it to the fabric either as a presoak before adding the dye, by mixing it with the dye, or by applying the soda ash afterwards. After-fixing is not very suitable for tie-dyeing, however, because the dye will run and bleed in the soda ash before it completes setting. It is better to use the soda ash first, before the dye.
If you have all-purpose dye and are intending to use it for tie-dyeing, please change your mind. Return the unopened packages of all-purpose dye to the store, or throw it away. It can only give poor results in tie-dyeing. You will get vastly better results if you use cool water fiber reactive dyes, made by Jacquard Products, Rainbow Rock, Tulip, Dylon, or Dritz. Rit is a hot water dye and should be applied only in scalding hot water, not at room temperature; see the instructions under "How can I tie dye with RIT dye?". If you've only used Rit dye in the past, you will be amazed at how much richer and brighter are the colors that Procion MX dyes produce, and how much longer they last in the laundry.
Vinegar is useful only when you are dyeing wool or silk with acid dyes. Wool and silk are chemically very different from cotton, so they can be used with vinegar, although cotton cannot. You can use Rit dye to tie-dye wool, silk, or nylon, if you presoak the fabric in vinegar, and follow the dyeing by wrapping the still-wet dyed fabric up in plastic wrap and steaming it in a covered pot over boiling water for at least half an hour, much as you would steam vegetables. Heat-setting is required if you are using Rit dye. If you use a cooking pot to immersion dye with Rit dye, do not reuse the pot for cooking food, because Rit dye is not safe for use on food preparation utensils and equipment.
(Please help support this web site. Thank you.)
Sunday, May 04, 2008
Procion MX dye in Italy?
Name: Fabio
Message: Hello!
My name is Fabio, you answered me some time ago on the itiedye forum, suggesting me the books of Ann Johnston. I've bought 3 of her books, and actually I'm reading DYE PAINTING! A really good book, thanks!
I've read the pages of the sources for dyeing supplies but cannot find any local vendor for the PROCION MX and other stuff.
Another thing, who can be a wholeseller of Procion MX and other stuff? I believe could be a good idea to sell the stuff for dyeing here in italy, with an online shop, what do you think?
I'm glad you are enjoying Ann Johnston's books. They are among my favorites.
You will probably not be surprised that I do not have any information about any Italian retailer for Procion MX type dyes, though I know of suppliers in several other European countries. Perhaps it would in fact be a good idea for you to consider selling them yourself. There are different ways you could import and sell Procion MX type dyes. I have not been involved in dye sales, myself, but some idea of who is selling dyes from what sources may be helpful to you to start with.
Probably the easiest way to become a retailer for these dyes would be to do as several companies have done, in various countries including Germany, the UK, and New Zealand, and become a distributer for the US company Jacquard Products, which sells Procion MX and other dyes in jars. The prices are higher this way than if you repackage barrels of dye, but you avoid the mess and physical dangers of spending a lot of time weighing out dye powders, which is a major consideration. I don't know what the practicalities may be. Contact Jacquard Products via their web site, or the phone numbers listed on their site. Look through the dye sellers in Europe and New Zealand on my list, or use Jacquard Products' "where to buy" store locator, to get an idea of how widely distributed these dyes are. You may also wish to contact PRO Chemical & Dye, to learn whether they would be able to supply dyes to you for reselling.
Dystar Textilfarben sells Levafix and Remazol warm-water dyes, as well as hot water dyes such as Procion H and Sirius direct dyes, in packages of five kilograms and up. Although they own the trademark for the Procion name, they no longer make Procion MX dyes (the dichlorotriazine dyes). I like to use Remazol dyes in place of Procion dyes, but Procion MX type dyes are better for room temperature use. Contact information:
Dylon sells different kinds of dyes, including more than one type of fiber reactive dye, in many countries throughout Europe, but their dyes are not as suitable for dye artists as other brands. The dyes themselves are good quality and highly suitable; the problem is that they are premixed in "fashion colors" which do not work nearly as well for the dye artist as the colors sold by Jacquard Products and other companies. It is very important for the dye artist to have access to the pure unmixed single-hue dyes, but these are not available from Dylon. In addition, all of the lines of dye besides Dylon Cold Dye have the auxiliary chemicals already mixed in, which makes alternative recipes impossible to follow. Their Dylon Cold Water dyes mostly contain dichlorotriazine dyes, the type in Procion MX type dyes, while Dylon Washing Machine Dyes, Dylon Permanent Dyes, and Dylon Hand Dyes contain mostly Drimarene K dyes and one Remazol dye. Their other line of dye, Dylon Multi Purpose Dye, contains all-purpose dyes, which are of very little interest for most dye artists.
There are many factories in the world that manufacture Procion MX type dyes, though they must use other trade names for their dichlorotriazine dyes. They often refer to them as "M Type" or "Cold Fix" dye, and you can verify which dye class by the Colour Index dye names they specify. There are many such dye factories in China or India. The one European factory I know of that sells dichlorotriazine dyes is Synthesia, in the Czech Republic. There can be quality issues with some dyes from some factories, so you'd have to test each shipment of dyes for solubility and perhaps freshness before investing in them. You would need to have some sort of facility in which to weigh the dyes out while using appropriate safety gear to avoid breathing in dye powder, which is likely to produce respiratory allergies and asthma if you do not take care. Although the prices of the dyes themselves, obtained in this way, would be far lower than for dyes purchased from a US company, the effort involved is vastly greater than that of reselling dye that is already packaged in appropriate-sized jars.
You will also need to look into customs requirements for importing dyes into your country. Some countries apply duty fees to imported dyes, which would make a considerable difference in what prices you will have to charge.
Please let me know if you decide to sell dyes.
(Please help support this web site. Thank you.)
Message: Hello!
My name is Fabio, you answered me some time ago on the itiedye forum, suggesting me the books of Ann Johnston. I've bought 3 of her books, and actually I'm reading DYE PAINTING! A really good book, thanks!
I've read the pages of the sources for dyeing supplies but cannot find any local vendor for the PROCION MX and other stuff.
Another thing, who can be a wholeseller of Procion MX and other stuff? I believe could be a good idea to sell the stuff for dyeing here in italy, with an online shop, what do you think?
I'm glad you are enjoying Ann Johnston's books. They are among my favorites.
You will probably not be surprised that I do not have any information about any Italian retailer for Procion MX type dyes, though I know of suppliers in several other European countries. Perhaps it would in fact be a good idea for you to consider selling them yourself. There are different ways you could import and sell Procion MX type dyes. I have not been involved in dye sales, myself, but some idea of who is selling dyes from what sources may be helpful to you to start with.
Probably the easiest way to become a retailer for these dyes would be to do as several companies have done, in various countries including Germany, the UK, and New Zealand, and become a distributer for the US company Jacquard Products, which sells Procion MX and other dyes in jars. The prices are higher this way than if you repackage barrels of dye, but you avoid the mess and physical dangers of spending a lot of time weighing out dye powders, which is a major consideration. I don't know what the practicalities may be. Contact Jacquard Products via their web site, or the phone numbers listed on their site. Look through the dye sellers in Europe and New Zealand on my list, or use Jacquard Products' "where to buy" store locator, to get an idea of how widely distributed these dyes are. You may also wish to contact PRO Chemical & Dye, to learn whether they would be able to supply dyes to you for reselling.
Dystar Textilfarben sells Levafix and Remazol warm-water dyes, as well as hot water dyes such as Procion H and Sirius direct dyes, in packages of five kilograms and up. Although they own the trademark for the Procion name, they no longer make Procion MX dyes (the dichlorotriazine dyes). I like to use Remazol dyes in place of Procion dyes, but Procion MX type dyes are better for room temperature use. Contact information:
DyStar Italia S.r.l.
Via delle Groane 126
20024 Garbagnate Milanese (MI)
Tel.: ++39-02-99 44 01
Fax: ++39-02-99 02 83 91
DyStar.Italy@DyStar.comDylon sells different kinds of dyes, including more than one type of fiber reactive dye, in many countries throughout Europe, but their dyes are not as suitable for dye artists as other brands. The dyes themselves are good quality and highly suitable; the problem is that they are premixed in "fashion colors" which do not work nearly as well for the dye artist as the colors sold by Jacquard Products and other companies. It is very important for the dye artist to have access to the pure unmixed single-hue dyes, but these are not available from Dylon. In addition, all of the lines of dye besides Dylon Cold Dye have the auxiliary chemicals already mixed in, which makes alternative recipes impossible to follow. Their Dylon Cold Water dyes mostly contain dichlorotriazine dyes, the type in Procion MX type dyes, while Dylon Washing Machine Dyes, Dylon Permanent Dyes, and Dylon Hand Dyes contain mostly Drimarene K dyes and one Remazol dye. Their other line of dye, Dylon Multi Purpose Dye, contains all-purpose dyes, which are of very little interest for most dye artists.
There are many factories in the world that manufacture Procion MX type dyes, though they must use other trade names for their dichlorotriazine dyes. They often refer to them as "M Type" or "Cold Fix" dye, and you can verify which dye class by the Colour Index dye names they specify. There are many such dye factories in China or India. The one European factory I know of that sells dichlorotriazine dyes is Synthesia, in the Czech Republic. There can be quality issues with some dyes from some factories, so you'd have to test each shipment of dyes for solubility and perhaps freshness before investing in them. You would need to have some sort of facility in which to weigh the dyes out while using appropriate safety gear to avoid breathing in dye powder, which is likely to produce respiratory allergies and asthma if you do not take care. Although the prices of the dyes themselves, obtained in this way, would be far lower than for dyes purchased from a US company, the effort involved is vastly greater than that of reselling dye that is already packaged in appropriate-sized jars.
You will also need to look into customs requirements for importing dyes into your country. Some countries apply duty fees to imported dyes, which would make a considerable difference in what prices you will have to charge.
Please let me know if you decide to sell dyes.
(Please help support this web site. Thank you.)
Saturday, May 03, 2008
Can I redye a canvas boat cover that faded in the sun?
Name: Sal
Message: Hi I am hoping that I could dye my canvas boat cover. It is blue the sun basicly discolored the top I have read everything and stil I am unsure. Do you have suggestions for me?
This is a difficult problem. Is the canvas on your boat made of a natural fiber, such as cotton or hemp? If not, it will be difficult to dye. Also, any dye you can use will be far more susceptible to fading by the sun than dye that is added to acrylic fiber when it is still liquid, before it is spun or extruded into thread.
I can't recommend that you dye a polyester or acrylic canvas boat cover. The only dyes that work on these fibers require that you boil the fabric in the dye, which is unlikely to be practical for canvas the size of your boat cover. How large of a cooking pot would you need to invest in! Furthermore, you can dye acrylic only with special dyes, either basic dyes or disperse dyes, which can be purchased only by mail-order; polyester can be dyed only with disperse dyes. I do not like to recommend that beginning dyers use basic dyes, because some of them are more hazardous for the user; some basic dyes will even, on occasion, cause cancer years later for the user.
What may be your best bet, aside from getting a new boat cover made from Sunbrella® acrylic or another sun-resistant material, would be to paint your cover, instead of dyeing it. Normally we avoid using ordinary acrylic-based paints on fabric, unless they are specifically labeled as "fabric paints", because the paint will dry to a very stiff and scratchy finish, and because the paint might fail to penetrate and may peel off. By using a thin acrylic fabric paint, such as Setacolor paint or Jacquard Textile Paint, this problem can be avoided, but the cost of the paint may be too high for a large project such as yours, even when purchased in large money-saving quart-sized bottles. An alternative is to prepare your own fabric paint by mixing something called fabric medium with artists' acrylic paints, which will help the paint to penetrate the fabric. In any case, fabric paint will show wear from abrasion much sooner than dye will.
If you have a new boat cover made, the name brand Sunbrella® acrylic fabric, although very expensive, will last much longer in the sun, because the fiber is solution dyed with light-resistant pigments or dyes. Beware of counterfeit fabrics or non-sun-resistant fabrics, which will fade far more quickly.
(Please help support this web site. Thank you.)
Message: Hi I am hoping that I could dye my canvas boat cover. It is blue the sun basicly discolored the top I have read everything and stil I am unsure. Do you have suggestions for me?
This is a difficult problem. Is the canvas on your boat made of a natural fiber, such as cotton or hemp? If not, it will be difficult to dye. Also, any dye you can use will be far more susceptible to fading by the sun than dye that is added to acrylic fiber when it is still liquid, before it is spun or extruded into thread.
I can't recommend that you dye a polyester or acrylic canvas boat cover. The only dyes that work on these fibers require that you boil the fabric in the dye, which is unlikely to be practical for canvas the size of your boat cover. How large of a cooking pot would you need to invest in! Furthermore, you can dye acrylic only with special dyes, either basic dyes or disperse dyes, which can be purchased only by mail-order; polyester can be dyed only with disperse dyes. I do not like to recommend that beginning dyers use basic dyes, because some of them are more hazardous for the user; some basic dyes will even, on occasion, cause cancer years later for the user.
What may be your best bet, aside from getting a new boat cover made from Sunbrella® acrylic or another sun-resistant material, would be to paint your cover, instead of dyeing it. Normally we avoid using ordinary acrylic-based paints on fabric, unless they are specifically labeled as "fabric paints", because the paint will dry to a very stiff and scratchy finish, and because the paint might fail to penetrate and may peel off. By using a thin acrylic fabric paint, such as Setacolor paint or Jacquard Textile Paint, this problem can be avoided, but the cost of the paint may be too high for a large project such as yours, even when purchased in large money-saving quart-sized bottles. An alternative is to prepare your own fabric paint by mixing something called fabric medium with artists' acrylic paints, which will help the paint to penetrate the fabric. In any case, fabric paint will show wear from abrasion much sooner than dye will.
If you have a new boat cover made, the name brand Sunbrella® acrylic fabric, although very expensive, will last much longer in the sun, because the fiber is solution dyed with light-resistant pigments or dyes. Beware of counterfeit fabrics or non-sun-resistant fabrics, which will fade far more quickly.
(Please help support this web site. Thank you.)
Friday, May 02, 2008
Can I overdye green coveralls to make them blue?
Name: Steve
Message: I have a pair of 80/20 cotton poplin/nylon coveralls that are (ugh) industrial mechanic green. I wish to make them blue. Can I overdye for the desired effect, or must I remove the existing color first?
You can't dye from a darker color to a lighter one, because dye is transparent. Although you can over dye blue with yellow to make green, you cannot overdye green to make blue, unless the green is very pale and your desired blue very bright or dark. If the green is light enough, you can overdye it to make a dark navy blue.
Beware of chlorine (hypochlorite) bleach. Although bleaching sounds like an attractive idea because it might remove the existing color, it is highly damaging to nylon. There are other chemicals that you can use to remove dye that are more gentle to synthetic fibers such as nylon. Any of the "reducing" type of discharge chemical can be used; avoid any "oxidative" discharge chemical. See "What chemicals can be used to remove dye?".
The easiest reductive discharge chemical to find in the US is Rit Color Remover, which can be purchased at almost any pharmacy or grocery store. Unlike all-purpose dye, Rit Color Remover is an excellent product. It works best if used in a large nonreactive coking pot, but it's a lot easier to use the washing machine with hot tap water, so try that first. You will probably need several boxes of Color Remover for one washing machine load. Follow the instructions printed inside the box closely.
Not all dyes can be removed, but there is absolutely no way to predict whether this will be a problem for your industrial mechanic green coveralls. It's certainly worth a try. The lighter you can get the dye color, the easier it will be to change to a different color. If you can't lighten the color, then black may be your only option for a color change.
(Please help support this web site. Thank you.)
Message: I have a pair of 80/20 cotton poplin/nylon coveralls that are (ugh) industrial mechanic green. I wish to make them blue. Can I overdye for the desired effect, or must I remove the existing color first?
You can't dye from a darker color to a lighter one, because dye is transparent. Although you can over dye blue with yellow to make green, you cannot overdye green to make blue, unless the green is very pale and your desired blue very bright or dark. If the green is light enough, you can overdye it to make a dark navy blue.
Beware of chlorine (hypochlorite) bleach. Although bleaching sounds like an attractive idea because it might remove the existing color, it is highly damaging to nylon. There are other chemicals that you can use to remove dye that are more gentle to synthetic fibers such as nylon. Any of the "reducing" type of discharge chemical can be used; avoid any "oxidative" discharge chemical. See "What chemicals can be used to remove dye?".
The easiest reductive discharge chemical to find in the US is Rit Color Remover, which can be purchased at almost any pharmacy or grocery store. Unlike all-purpose dye, Rit Color Remover is an excellent product. It works best if used in a large nonreactive coking pot, but it's a lot easier to use the washing machine with hot tap water, so try that first. You will probably need several boxes of Color Remover for one washing machine load. Follow the instructions printed inside the box closely.
Not all dyes can be removed, but there is absolutely no way to predict whether this will be a problem for your industrial mechanic green coveralls. It's certainly worth a try. The lighter you can get the dye color, the easier it will be to change to a different color. If you can't lighten the color, then black may be your only option for a color change.
(Please help support this web site. Thank you.)
Thursday, May 01, 2008
How do I clean a batik before reframing it?
Name: carmen
Message: I have a batik....on cotton fabric for many years, framed and now is showing fading or dust? how do I clean it before I get it framed again with a more newer modern frame? I got it from an art student, using the wax....thanks a million because I like it and have been with me for quite a long time!
It's hard to tell what you should do.
If the dye used in the batik was fixed properly, which is not hard to do, then you can just wash it. The best dyes to use for batik are cool water fiber reactive dyes, such as Procion; if the artist who made your batik used these dyes and knew anything about dyeing, then the dye will not run or fade, even if you use hot water and a washing machine.
If, on the other hand, the batik was made with improperly fixed dye, then getting the batik wet could cause the dyes to run. This is a particular problem with indigo-dyed textiles from Vietnam, Guatemala, or some parts of Africa (to name the three regions about which people email me most, after buying products made with unfixed dyes there).
If the dyes used in your batik were not applied or washed out properly, then the safest thing to do would be to thoroughly vacuum to remove dust, and then reframe without washing.
Since you say your batik was made by an art student, it is probably safe to wash the batik, at least in cool water. Try rubbing water on an inconspicuous corner first, or dampening the cloth in that inconspicuous corner and rubbing it with a white rag. If no color transfers, you will probably be safe with washing.
(Please help support this web site. Thank you.)
Message: I have a batik....on cotton fabric for many years, framed and now is showing fading or dust? how do I clean it before I get it framed again with a more newer modern frame? I got it from an art student, using the wax....thanks a million because I like it and have been with me for quite a long time!
It's hard to tell what you should do.
If the dye used in the batik was fixed properly, which is not hard to do, then you can just wash it. The best dyes to use for batik are cool water fiber reactive dyes, such as Procion; if the artist who made your batik used these dyes and knew anything about dyeing, then the dye will not run or fade, even if you use hot water and a washing machine.
If, on the other hand, the batik was made with improperly fixed dye, then getting the batik wet could cause the dyes to run. This is a particular problem with indigo-dyed textiles from Vietnam, Guatemala, or some parts of Africa (to name the three regions about which people email me most, after buying products made with unfixed dyes there).
If the dyes used in your batik were not applied or washed out properly, then the safest thing to do would be to thoroughly vacuum to remove dust, and then reframe without washing.
Since you say your batik was made by an art student, it is probably safe to wash the batik, at least in cool water. Try rubbing water on an inconspicuous corner first, or dampening the cloth in that inconspicuous corner and rubbing it with a white rag. If no color transfers, you will probably be safe with washing.
(Please help support this web site. Thank you.)


