« 2011 September | Main | 2011 July »
Friday, August 26, 2011
Ddo you where I can find safe felt? I'm looking to source my supplies as natural, local and safe for children or pets if accidentaly ingested.
Country or region: USA
Message: Hello, do you know where I can find safe felt? I'm looking to source my supplies as natural, local and safe for children or pets if accidentaly ingested. I have looked so many places and have come up with nothing. I went to Hobby Lobby today and bought acrylic felt to practice with. It is difficult to hand cut and is stretching out.
I would think that the main danger with felt would be as a choking hazard, if there are pieces small enough for a baby to detach and chew on. Perhaps a synthetic-fiber felt might be safer for babies than natural-fiber felt, if it is more difficult to chew into pieces! Small pieces of felt, or pieces that can be chewed off, represent a hazard to any child or pet who chews on them, regardless of what they are made from. Long narrow strips of fabric, string, or ribbon can be particularly hazardous or even fatal to pets who swallow them.
Wednesday, August 24, 2011
I'm interested in dyeing scrub tops Name: Brenda
If you are successful with finding dyeable scrubs, please let me know how they work out for you. Be sure to use a good fiber reactive dye, such as Procion MX dye; don't use an all-purpose dye, such as Rit, for tie-dyeing.
(Please help support this web site. Thank you.)
Can you recommend a fixative other than Retayne to stop Rit dye on nylon from bleeding?



Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Buy from
Paradise Fibers


Color for Science, Art and Technology, Volume 1, edited by Kurt Nassau
This technical book explains many features of modern dye chemistry, including the function of syntans as acid dye fixatives.


Country or region: NW USA
Message: I am using Rit Dye to color nylon and I am having trouble to stabilize the dye from bleeding after dying. I tried washing in Retayne, but the color still bleeds afterwards. Some German contacts are recommending Lavegal. Are you familiar with this product? Do you know if this or a comparable product is available in the USA? Thanks for your consideration!!!
Monday, August 22, 2011
I would like the white to actually remain as white as possible...is there a trick to doing this?
Country or region: Spokane Washington, USA
Message: Well, I am new to the art of tye dying . . .have had a blast exploring the artistic side of this art form. I do however have a question regarding keeping white areas truly white if that is possible. After untying each project, it is such a cool discovery of the beautiful piece of art just created. There are times when working with white t-shirts, that I would like the white to actually remain as white as possible...is there a trick to doing this??? I would really appreciate your response....Thanks a bunch
You will probably find it necessary to repeat the final hot water wash twice; in some cases, three repetitions are needed. When you are done, your white areas, wherever you had no dye at the end of your "batching" time for the dye reaction, will be white again.
(Please help support this web site. Thank you.)
Sunday, August 21, 2011
I'm interested in dyeing satin back taffeta bridesmaid dresses
Country or region: NY, USA
Message: I'm interested in dyeing satin back taffeta bridesmaid dresses from Tiffany box color to brown or black. Wanted to know if possible, cost to have you do it and a turn around time. I appreciate and thank you for your time to respond.
Friday, August 19, 2011
What makes indigo so special, compared to other blue dyes?


A Handbook of Indigo Dyeing,
by Vivien Prideaux
This book provides an excellent introduction to many basic methods of shibori dyeing, in addition to easy-to-follow illustrated recipes for using indigo.




Shibori: The Inventive Art of Japanese Shaped Resist Dyeing
This is the classic work on traditional Japanese shibori, a must for any serious shibori artist, though not intended to be a beginner's handbook.


Country or region: U.S.
Message: I am going to try dyeing some shibori. What blue color of Procion MX will approximate indigo most closely? What is it about indigo dye that makes it so special when you compare it to other blue dyes?
Thursday, August 18, 2011
Should I have a dry-clean-only dress cleaned before I dye it, or afterwards?
Country or region: United States
Message: My daughter has a silk dress she wants dyed. It is a dry-clean-only dress. Should I have it cleaned before I dye it, or afterwards?
Wednesday, August 17, 2011
Country or region: Cape Cod, MA
Tuesday, August 16, 2011
Is it possible to dye clothing made of cupro?
Country or region: USA
Message: Hello,
I did a little research per Google, Cupro is a form of rayon. So does this mean I can use a dye that works on rayon material? Also, what would be the best method to insure an even dark black color?
Thursday, August 11, 2011
I want to dye basketball socks, orange on a royal blue sock. Can I dye the whole sock, or do I have to spot dye just the white areas?
Country or region: Texas
Message: I want to dye some Nike ELITE basketball socks. My son wants the white parts to be orange on a royal blue sock. Can I dye the whole sock, or do I have to spot dye just white areas. Thanks and appreciate the advice.
Monday, August 08, 2011
Is it possible to mix some of the Jacquard Red label dyes into the Green label to get slightly more intense colors, without steam-setting the dye afterwards?
Country or region: USA
Message: I read where you said the Jacquard Red Label dyes are twice as concentrated as the Green Label. Is it possible to mix some of the Jacquard Red label dyes into the Green label to get slightly more intense colors? I prefer to use the dyeset instead of steaming.
I do have several old scarves made with the Red Label & old version of dyeset, BEFORE the Green label was even invented. The colors are so much more intense & luminous.
What if I do a vinegar soak before painting on a Green + Red label mixture?
Thursday, August 04, 2011
How can you tell to which class of acid dye a particular dye belongs?


Wool Dyeing
Edited by David M. Lewis, 1992.
Highly technical and thorough, this book explains all aspects of wool dyeing in a manner understandable by textile professionals and advanced science students.


Victor B. Ivanov's

Reactive Dyes in Biology and Medicine
Explains use of reactive dyes for staining proteins or carbohydrates


Waring and Hallas's


The Chemistry and Application of Dyes (Topics in Applied Chemistry)
includes recipes for synthesizing reactive dyes


John Shore's

Cellulosies Dyeing
Useful information about the chemistry of reactive dyes, and other dye types used for cotton and other plant fibers


Country or region: Switzerland
Message: I would just like to ask a questions about the different types of acidic dyes: How do you tell that a dye is of which type? The dye in particular I am enquiring about specifically is Acid Blue 93. I think it is a acid-milling dye, but I am not sure. Thank you!
(from Chemblink.com, at http://www.chemblink.com/products/28983-56-4.htm)
Tuesday, August 02, 2011
Will Retayne set a dye originally intended to be washed out?
Country or region: Nebraska, USA
Message: Will Retayne set a dye originally intended to be washed out? I bought an old feed sack (off-white with colored logo) with printed instructions for washing out the logo. The instructions are to wash in water greater than 150 degrees. I want to retain the logo and use fabric in a quilt that will be rarely washed. How can I prepare and care for this fabric to preserve the print?
Monday, August 01, 2011
Is there a dye pen I could use to "color" over the embroidery?
Country or region: USA
Message: Hi, I own a beautifully embroidered pillow that I would like to change a color on. Part of the embroidery is a rusty orange which completely clashes with the red in my room. Is there a dye pen I could use to "color" over the rusty orange, making it more in the red family? What do you recommend? Thank you for your help!
Alternatively, you might try painting on the rusty orange with a fabric paint, using a fine natural-fiber brush. Like fabric markers, fabric paints are much more suitable for this use than other paints, which would leave the embroidery stiff and hard. One fabric paint, Neopaque, which is made by Jacquard Products, is opaque and therefore can be used for more marked color changes; most fabric paints, and all of the fabric markers I know, are transparent, and allow some of the original color to show through. Whether you choose fabric paints or fabric markers, be sure to practice first to find how easy it is for you to control the marks that you make.
(Please help support this web site. Thank you.)
Ddo you where I can find safe felt? I'm looking to source my supplies as natural, local and safe for children or pets if accidentaly ingested.
Name: Bree
Country or region: USA
Message: Hello, do you know where I can find safe felt? I'm looking to source my supplies as natural, local and safe for children or pets if accidentaly ingested. I have looked so many places and have come up with nothing. I went to Hobby Lobby today and bought acrylic felt to practice with. It is difficult to hand cut and is stretching out.
Wool is the fiber from which felt is traditionally made. Customers who are interested in natural materials will prefer wool felt over felt made of acrylic or polyester, as will anyone who enjoys the tactile qualities of natural fibers. Wool is far more beautiful than acrylic or polyester. It's also far easier to dye wool felt yourself than any of the synthetic felts. Do a search online for "natural wool felt". One source that pops up in response to such a search is Michigan-based Weir Crafts, which sells felt made of 100% wool, a US-made 30% rayon/70% wool, and a US-made wool of 50% wool plus 50% bamboo rayon. Their products include an Oeko-Tex-certified natural undyed 100% wool felt. I doubt you will be able to find any natural fibers at Hobby Lobby, and it's likely that most of their products are made in China, so, ironically, you may have to resort to buying online in order to get more locally-sourced products.
If you want the dyes in your felt to be 100% safe for ingestion, you can buy undyed wool felt and dye it with Kool-aid or another synthetic food coloring. (Rayon cannot be dyed with food coloring.) These food dyes have been tested for safety even when consumed in huge quantities, so the tiny amounts a baby might extract from felt by chewing on it would certainly be safe, assuming that it is not possible to bite off chokable pieces. For dyeing rayon and other plant-based fibers, synthetic fiber-reactive dyes, such as Procion dyes, are very safe for babies, because they form such a tight bond to the fiber that, unlike other types of dyes, they will not come out of the fabric when the fabric is sucked on.
For customers who find the idea of even food-safe dyes unaesthetic, if the dyes are synthesized from petroleum sources, you might consider natural dyes. However, most natural dyes require mordanting with metal ions. All of the metal-based mordants are at least potentially toxic. The safest of all of the metal-based mordants for natural fibers is alum, but I would certainly not use it in baby clothing or toys, since my favorite synthetic Procion dyes, when properly used, are certainly much safer than alum, for babies' clothing and toys. You may see misleading references to "food grade" alum in some advertising, but in fact alum is not approved by the FDA for use in food. While it takes about an ounce of alum to kill an adult, smaller quantities are sufficient to kill a small child; quantities that are much smaller than the lethal dose can have some toxic effects and can be irritating to the skin and mouth, and thus are not suitable for anything a baby or pet might chew on.
Completely safe natural dyes that do not require a mordant do not retain their color nearly as long as other dyes, but there are occasions when they are the right answer, as long as you are honest about the impermanence of the dye in your package labeling. Turmeric and walnut are natural dyes that can be used without a mordant, though they may work better with one; turmeric is a light-sensitive bright yellow dye, which fades in sunlight, while walnut hulls produce brown. Cochineal is a bright red natural dye, made from cactus bugs, that is frequently used to color foods; while it works best with a mordant, on wool it works reasonably well even without a mordant. Indigo is a wonderful natural dye that can be used without mordants on many different natural fibers, including felt; the harsh chemicals used to apply it are, unlike mordants, completely removed by washing. Unfortunately, indigo dye is considerably more difficult for a novice to use, but, if you have time to learn the complex process, you will find that the blue it produces is more wonderful than the colors produced by most other mordant-free natural dyes, and longer-lasting, as well. Note that most indigo is synthesized from petroleum sources, but it is possible to buy natural, plant-derived indigo, if you check the label carefully. Items dyed with synthetic indigo are completely safe for babies to use, once they've been properly washed, but the plant-derived indigo may be more attractive in concept to your customers.
If you want truly local felt, you may be able to find crafters in your state who make felt. Or, you might be able to find wool grown in your own state with which to make your own felted toys. (Try going to your local county fair to try to meet someone who raises sheep.) Felting is a very interesting way to make not only flat fabric, but also balls, dolls, and other simple toys for children. A good way to decide whether you like this process might be to begin with a felt kit; I see some nice-looking felt kits for sale on Etsy and on Artfire.com.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Wednesday, August 24, 2011
I'm interested in dyeing scrub tops Name: Brenda
Country or region: US
Message: Hello. Your web presence has answered many dye questions for me. Thanks.
I'm interested in dyeing scrub tops for a charity fund raising project. Availability? Prices?
The hardest part about dyeing scrubs may be finding the blanks to dye. Look for white 100% cotton, if you can; a cotton-rich blend, such as 80% cotton, will dye pretty well, but the standard 65% polyester/35% cotton will produce only very pale colors.
If you happen to see scrubs that are mostly bamboo, or another kind of rayon, that would probably be a good choice. Polyester is to be avoided because it's so much more difficult to dye.
Avoid anything that's labeled "stain resistant", because stain-resistant clothing resists dyes, as well.
Dharma Trading Company used to sell dyeable 100% cotton scrubs, but most of their sizes have been out of stock for some time. Try a web search for 'white "100% cotton" scrubs'. It looks as though a company named Comfort Scrubs may be selling a suitable 100% cotton white scrub top, for a very reasonable $7.50, but I don't know anyone who's tried it.
If you happen to see scrubs that are mostly bamboo, or another kind of rayon, that would probably be a good choice. Polyester is to be avoided because it's so much more difficult to dye.
Avoid anything that's labeled "stain resistant", because stain-resistant clothing resists dyes, as well.
Dharma Trading Company used to sell dyeable 100% cotton scrubs, but most of their sizes have been out of stock for some time. Try a web search for 'white "100% cotton" scrubs'. It looks as though a company named Comfort Scrubs may be selling a suitable 100% cotton white scrub top, for a very reasonable $7.50, but I don't know anyone who's tried it.
If you are successful with finding dyeable scrubs, please let me know how they work out for you. Be sure to use a good fiber reactive dye, such as Procion MX dye; don't use an all-purpose dye, such as Rit, for tie-dyeing.
(Please help support this web site. Thank you.)
Can you recommend a fixative other than Retayne to stop Rit dye on nylon from bleeding?
Name: Alex
—ADVERTISEMENTS—

Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Buy from
Paradise Fibers
Washfast Acid dyes
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!

Color for Science, Art and Technology, Volume 1, edited by Kurt Nassau
This technical book explains many features of modern dye chemistry, including the function of syntans as acid dye fixatives.

Country or region: NW USA
Message: I am using Rit Dye to color nylon and I am having trouble to stabilize the dye from bleeding after dying. I tried washing in Retayne, but the color still bleeds afterwards. Some German contacts are recommending Lavegal. Are you familiar with this product? Do you know if this or a comparable product is available in the USA? Thanks for your consideration!!!
I think you'd be much better off choosing a better dye, instead of trying to find a special fixative to improve the washfastness of the poor-quality dye you've been using.
Retayne is a cationic dye fixative, which does help to fix poorly washfast dyes, that is, dyes that tend to bleed when wet. It is designed for use on direct dyes that have been used on cotton and similar fibers. While cationic dye fixatives work by sticking, with their positive charge, to negatively-charged dyes (including acid dyes), there are also acid dye fixatives that work by a different mechanism. For example, one form of acid dye fixative contains synthetic tannins, or syntans, which are negatively-charged compounds with high molecular weights, used to form a surface layer on the fiber, after dyeing, to help seal dye molecules inside the fiber. I have not been able to find much information about Lavegal (most references appear to concern an entirely different product used in galvanizing metal), but, in general, dyeing assistants used to improve the performance of acid dyes on nylon appear to be available in vast quantities suitable only for large-scale industrial use, so it's unlikely you will be able to obtain reasonably small quantities. (Here's a link to the only meaningful information I could find on Lavegal FL.) Anyway, I think it makes more sense for you to concentrate on choosing a better dye.
All-purpose dyes, such as Rit, are known for being exceptionally poorly washfast, which means that they tend to bleed in the laundry or when wet. All-purpose dyes contain a mixture of leveling acid dye, for dyeing wool and nylon, and direct dye, for dyeing plant-based fibers such as cotton. Both of these types of dye are notable for their very low permanence. Leveling acid dye is, in fact, the most poorly washfast form of acid dye. Leveling acid dye is a good choice when you don't care how much the dye bleeds, but when you want a very smooth and even solid color. If you don't want your dye to bleed, you should choose an entirely different sort of acid dye.
A better choice for nylon would be the Washfast Acid dyes, also called Nylomine dyes for their good performance on nylon. Most of the Washfast Acid dyes are acid milling dyes, which are significantly more washfast than leveling acid dyes. The Washfast Acid Jet Black contains a premetallized acid dye; premetallized acid dyes are even more resistant to bleeding the acid milling dyes. The amount of acid required for these dyes is not as great as that required for the acid leveling dyes; acid leveling dyes are also known as "strong acid" dyes, because of the very low pH they require. (Note that dyeing nylon with Rit dye requires heating the dye and the nylon together in the presence of an acid; see my page, About All Purpose Dye, for more details on how much acid to use.)
Another good choice for nylon would be the Lanaset dyes, which includes both premetallized and reactive dyes. These dyes are more expensive per pound, but less dye is needed. They are significantly more washfast than the Washfast Acid dyes, at least on wool; they are so exceptionally washfast that they are tested by washing in hot water at 140°F, unlike other acid dyes whose washfastness is tested by washing in cooler water, at 105°F.
Any other sort of acid dye you can buy will give better performance than acid leveling dyes. Any acid dye you choose will be better than the dyes contained in the all-purpose dye you've been using.
You can buy these superior acid dyes online from several different dye suppliers. In the US, suppliers for Washfast Acid Dyes include PRO Chemical & Dye and Paradise Fibers; suppliers for Lanaset dyes in the US include PRO Chemical & Dye, Paradise Fibers, and Earth Guild. Dharma Trading Company also sells a number of leveling acid dyes, acid milling dyes, and premetallized dyes. Washfast Acid dyes and Lanaset dyes are available in Vancouver from Maiwa Handprints. Avoid the leveling acid dyes, and go for acid milling dyes, premetallized dyes, or Lanaset dyes.
Incidentally, some people assume that all-purpose dye is the cheapest choice, when in fact all-purpose dye is not very economical to use, since each package contains a great deal of salt and detergent, and only a little dye. See my Dye Forum post, "comparison of dye costs". To dye one pound of nylon to a pale to medium with Rit dye requires approximately $2 worth of dye, whereas you can buy enough Washfast Acid dye to dye the same amount of fiber to a medium shade for just 55¢ (plus shipping), assuming you buy two-ounce packages. In the long run, the better performance alone is sufficient reason to stop using all-purpose dyes, but it's nice that we can also save money by doing so.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Monday, August 22, 2011
I would like the white to actually remain as white as possible...is there a trick to doing this?
Name: AJ
Country or region: Spokane Washington, USA
Message: Well, I am new to the art of tye dying . . .have had a blast exploring the artistic side of this art form. I do however have a question regarding keeping white areas truly white if that is possible. After untying each project, it is such a cool discovery of the beautiful piece of art just created. There are times when working with white t-shirts, that I would like the white to actually remain as white as possible...is there a trick to doing this??? I would really appreciate your response....Thanks a bunch
The trick to keeping your white areas really white is to avoid backstaining.
It's impossible to avoid backstaining if you don't use fiber reactive dyes, such as Procion dyes. People who do tie-dyeing with all-purpose dyes, such as Rit dye, invariably see their other colors run onto the white when the shirt is washed.
To avoid permanent backstaining with Procion dyes, it's very important to allow more than enough time, in a warm place with moisture present, for the reaction between the dye and the fiber to complete. If you wait long enough, then all of the reactive dye molecules will have time to react, either with the fiber in your fabric, or with the water itself. Once all of the dye molecules have reacted, any dye that transfers from one part of the shirt to another will be unable to react again to form a permanent bond to the fiber, which means it can be washed out. Allow at least twelve hours, or longer, at a temperature of 70°F or higher, for this step, after applying your dyes (and the soda ash); maintain moisture for the entire time, either by wrapping each item in plastic, or by including urea in your dye mixtures (use one tablespoon, or 15 ml, of urea per cup, or 250 ml, of water).
After you've allowed more than enough time for all of the dye reactions, when you first rinse out the dye, you may see some color transfer from a dark section to a light section. Don't worry; this dye is not permanent, since you have already allowed the dyes to lose their ability to react with the fiber before this step.
Start your washing-out of the excess unattached dye by rinsing with cool water; some prefer to rinse carefully before untying, and continue to rinse by hand, but I like to untie everything and put many items into a single washing machine load, already full with cool water, for the first washing. In this cool water step, color may transfer temporarily.
The remainder of the unwanted dye then gets removed in the next step of washing. For this step, you must use HOT water. Many dyers like to use Synthrapol as the detergent at this step; it's a good choice, but it's not essential. What is essential is making sure that the water is hot enough. Put a thermometer into your water supply where it comes into the washing machine (or into your sink if you are washing by hand), and make sure that your water is 140°F or warmer. 140°F is the standard for water heaters in the US, but it is common to turn down the thermostats on water heaters to only 120°F in order to reduce the risk of scald injury. (If you turn up your water heater for your dye washing, be sure to let everyone in your household know to be careful, and don't forget to turn the thermostat back to your preferred setting when you are done.) The hotter the water is, the more efficiently it will remove the unattached excess dye. Fiber reactive dyes, such as Procion dye, bond so permanently to a natural fiber that even boiling water will not remove them; in fact, some dyers prefer to use nearly-boiling water for the most efficient washing out.
You will probably find it necessary to repeat the final hot water wash twice; in some cases, three repetitions are needed. When you are done, your white areas, wherever you had no dye at the end of your "batching" time for the dye reaction, will be white again.
My own washing machine, as a supposed "energy saver" feature, adds cold water to even the hot setting, resulting in water that is too cool for efficient dye wash-out (which results in a requirement for more wash cycles, thus canceling any savings). I turn off the cold water faucet that leads to the washing machine while I am filling the machine with hot water, then turn it back on afterwards.
Sometimes additional difficulties in washing out can be caused by washing with hard water. The calcium ion can cause the dyes to form larger complexes that are more inclined to cling to the fiber, in spite of the fact that they have not bonded to the fiber. To avoid this, if your water is hard, either use a water softener system, or add the water softener sodium hexametaphosphate to both your dye reactions and to your wash water. See "Dyeing with hard water: water softeners, distilled water, and spring water".
All of the above will keep your whites as white as possible. In order to have more white in your designs, you can thicken your dyes so that they do not creep as far into the fabric. See "Sodium alginate, Superclear, and other dye thickeners". It's often helpful to place additional ties or rubber bands to reinforce the first ones you place; it's especially helpful to add a firm rubber band or tie on top of hand-sewn resists.
(Please help support this web site. Thank you.)
Sunday, August 21, 2011
I'm interested in dyeing satin back taffeta bridesmaid dresses
Name: Gina
Country or region: NY, USA
Message: I'm interested in dyeing satin back taffeta bridesmaid dresses from Tiffany box color to brown or black. Wanted to know if possible, cost to have you do it and a turn around time. I appreciate and thank you for your time to respond.
Whether you can dye these dresses or not depends on what they're made of, and on whether they are washable. "Satin" and "taffeta" can be made from many different fibers. A really high quality satin or taffeta will be made of natural silk, but, if you don't know what the dresses are made of, they are probably polyester or nylon. Please read the following page on my web site: "How can I dye satin or charmeuse?".
I am not willing to dye your dresses for you, but you can contact one of the garment dyers on the page, "Where can I find someone to dye my clothing for me?". Metro Dyeing Service is probably your best bet, since they are located in New York. Contact them to find out whether your dresses are dyeable at all, and, if so, how much dyeing them will cost.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Friday, August 19, 2011
What makes indigo so special, compared to other blue dyes?
Name: Kathy
—ADVERTISEMENTS—

A Handbook of Indigo Dyeing,
by Vivien Prideaux
This book provides an excellent introduction to many basic methods of shibori dyeing, in addition to easy-to-follow illustrated recipes for using indigo.


Shibori: The Inventive Art of Japanese Shaped Resist Dyeing
This is the classic work on traditional Japanese shibori, a must for any serious shibori artist, though not intended to be a beginner's handbook.

Country or region: U.S.
Message: I am going to try dyeing some shibori. What blue color of Procion MX will approximate indigo most closely? What is it about indigo dye that makes it so special when you compare it to other blue dyes?
The most special thing about indigo is that, for thousands of years, it was the only good blue dye in existence. Until the discovery of Prussian blue in the mid-eighteenth century, indigo was the only good blue dye available in most of the world, though in the seventeenth century the introduction of logwood to Europe provided a less permanent alternative blue. The indigo dye molecule is produced by some fifty different plant species, worldwide; its unique ability to act as a permanent blue textile dye has been discovered repeatedly by different cultures around the world. Plant-derived indigo is still available, though the majority of indigo these days is synthesized from petrochemicals.
Unlike most natural dyes, indigo can be used without metal-based mordants such as alum, tin, or chrome, which stay in the fabric after dyeing, and which are toxic if ingested. Unlike mordants, the chemicals that are used with indigo are washed out completely after the dyeing process. Indigo is notable for being both more light-resistant and more wash-resistant than almost all other natural dyes. It's closely related to the famed Tyrian purple dye of antiquity, made by shellfish.
There are several different processes for applying vat dyes such as indigo, using fast-acting modern synthetic chemicals or slow old-fashioned fermentation vats, but all cause the dye to be chemically reduced to render it soluble, so that it penetrates inside textile fibers. Exposure to air then chemically oxidizes the dye, returning it to its insoluble state, so that dye molecules inside the textile fibers become permanently trapped. Until the invention of fiber reactive dyes, this was the best mechanism for attaching a dye to a textile fiber permanently. Other older types of dyes, the mordant dyes and direct dyes, are far more prone to quickly fading in the laundry.
Ironically, one of the most-prized properties of indigo in the fashion industry now is the result of inferior dyeing practices. If indigo is applied improperly, most of it will deposit on the outside of textile fibers, instead of penetrating further within them. Since much of the indigo in denim fabric is allowed to remain on the outside of the individual cotton fibers, in a dyeing fault known as "ring dyeing", it is easily worn off, so that a small amount of abrasion results in an exaggerated appearance of age. Unfortunately, some commercial denims, even those used in very expensive blue jeans, are so poorly dyed that a significant amount of blue dye is completely unattached, so that it crocks (that is, rubs off even when dry) onto other clothing, shoes, and purses, sometimes ruining them. An expert dyer can apply indigo so that crocking is not a big problem, but evidently some major clothing retailers do not care.
Fiber reactive dyes, such as Procion MX, are much easier to apply than indigo, and they have greater washfastness and, in most cases, equally good lightfastness. Vat dyes tend to have higher resistance to fading from light than other classes of dye, often ranking as high as 7 or 8 on a scale of 1 to 8; however, indigo is listed by a manufacturer as having a lightfastness rating of only 5, less than that of Procion blue MX-R. (See my page, "Lightfastness of Different Types of Dyes".) The main motivations for hand-dyers to use indigo are for historical reasons, and, for people who buy plant-sourced indigo, the satisfaction of using non-petroleum-based dyes.
The color indigo is a medium-bright blue. It is not nearly as bright as, say, the brilliant Remazol Blue R (reactive blue 19), because it absorbs light over a wider range of the visible spectrum, but it is brighter than some navy blue dyes. None of the unmixed ProcionMX dye colors exactly matches the hue of indigo. Procion Blue MX-7RX is too violet, while Procion Blue MX-G, Procion Navy MX-3R, Procion Blue MX-2G, Procion Blue MX-4GD, Procion Blue MX-3G, and Procion Turquoise MX-G are all too green. (See "Which Procion MX colors are pure, and which mixtures?".
I think that the closest match to indigo among the unmixed Procion MX blues would be Procion Blue MX-R, or reactive blue 4. Like indigo dye, it is a duller color than Procion Blue MX-G, and it is the only one of the Procion blue dyes that is neither greenish nor purplish. At a concentration of 4% OWD (that's four grams of dye per 100 grams of fabric), it looks very much like a medium indigo; at a weaker strength of 1.5%, Dharma calls it "sky blue", which looks like a paler dyeing with indigo.
For an even closer match from the Procion MX color range, you may prefer to choose a pre-mixed color, such as the "422N Indigo Blue" sold by PRO Chemical & Dye. Dharma's "PR130 Strong Navy" mixture has also been recommended as similar in color. Let me add that, much as I love true indigo dye, I like the color of Remazol Blue R even more, and also the cerulean blue Procion Blue MX-G. High concentrations of either of these unmixed dye colors produce fairly dark and yet glowing blues. They are both more blue than true indigo is, however.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, August 18, 2011
Should I have a dry-clean-only dress cleaned before I dye it, or afterwards?
Name: Charna
Country or region: United States
Message: My daughter has a silk dress she wants dyed. It is a dry-clean-only dress. Should I have it cleaned before I dye it, or afterwards?
If you can't wash it, you can't dye it. Dyeing always involves a lot of washing.
Many garments that are labeled "dry clean only" can in fact be washed, but some will disintegrate if you try it. You won't know which you have unless you try it. Wash the dress; if it survives, then you can consider dyeing it. If you're not willing to risk losing the dress, you should not try washing it, which means you can't dye it.
Before dyeing, you must wash the dress thoroughly to remove invisible stains and finishes that will prevent the dye from adhering evenly; after dyeing, you must wash to remove all unattached excess dye, as otherwise it will rub off on anything that touches the fabric. Neither of these can be done without washing the dress.
More posts on this topic:
Wednesday, August 17, 2011
Name: Barbara
Country or region: Cape Cod, MA
Message: Looking for a recipe to make PARCHMENT with Cushing dyes. I also want to make INDIGO. Thanks.
If you would like to mix your own dye colors, you might do better to choose another brand of dye, one whose makers intend for their dye colors to be mixed.
W. Cushing & Company intends for their dyes to be used as packaged, rather than for blending your own colors. They say that this is why there are forty different colors available among their direct dyes, and over ninety different colors among their acid dyes. They believe that you should be able to find the color you need among the various mixtures that they have already blended.
Good alternatives to Cushing Perfection Acid Dye, for dyeing protein fibers such as wool, include the Lanaset dyes and the WashFast Acid dyes. A superior alternative to Cushing Perfection Direct Dye, for dyeing plant-based fibers, is fiber reactive dye, such as Procion MX dyes. A nearby source for all of these dyes for you would be PRO Chemical & Dye, in Fall River, Massachusetts; they sell many different types of dye both locally and online.
If you contact W. Cushing, they will send you a color card at no charge. You can also see pictures of their color cards on their website; like all color cards viewed online, they are less reliable than the printed version, so it's a good idea to ask for the card. For a parchment-like color, among their acid dyes, Cushing carries "Old Ivory" and "Ecru"; among their direct dyes, there is no close match, so you would need to use a very dilute amount of a brown color, such as their "Light Brown". For an indigo-like color, among the Cushing direct dyes you might choose either "blue" or "navy blue", while the Cushing acid dyes include both "blue" and "royal blue".
Not all dyes are compatible for mixing with one another. It is best to mix your own colors from dyes that are promoted by the seller as being suitable for using together. The Cushing representative told me that, if you wish to mix your own Cushing Acid dyes, their best colors to use as mixing primaries for light colors are Canary, Cherry and Peacock, and, for dark colors, Yellow, Blue and American Beauty. For Cushing Direct dye, the recommended mixing primaries for light colors are Scarlet, Light Blue or Copenhagen Blue, and Yellow, while the recommended mixing primaries for darker hues would be Blue, Cardinal and Canary. A neutral color such as parchment will require some of each of the three primary colors; since it's a pale color, you should use a smaller amount of dye powder than usual, for a given amount of fiber.
If you are using Cushing Direct dyes, for plant fibers such as cotton, you will find fiber reactive dyes to be longer-lasting and less prone to fading in the laundry. For more information on mixing fiber reactive Procion dyes, see my FAQ answer page, "How can I mix Procion MX dyes to get specific colors?". Discussions of mixing various dye colors with different classes of dye can be found in the "Color Mixing" forum topic of the Dye Forum.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Tuesday, August 16, 2011
Is it possible to dye clothing made of cupro?
Name: Laura
Country or region: USA
Message: Hello,
I was wondering if it is possible to dye a pair of Cupro shorts? The shorts are a caramel color & I would love to dye them black. The tag says 100% cupro and dry clean only, but I have washed them on a delicate cold water cycle & they held up just fine.
I did a little research per Google, Cupro is a form of rayon. So does this mean I can use a dye that works on rayon material? Also, what would be the best method to insure an even dark black color?
Yes, cupro, or cuprammonium rayon, is a manufactured regenerated cellulosic fiber, essentially just another form of rayon, though more expensive to manufacture than viscose rayon. One popular brand name for cupro is Bemberg, produced in Italy. Since it's a form of rayon, it can be dyed with the same dyes that work on other rayons. (See "How to Dye Rayon".) The best dye for rayon is a cool water fiber reactive dye, such as Procion MX dye. (See "About Fiber Reactive Dyes".) Other dyes that will work include direct dyes (which require heat for application, and fade quickly in the laundry), vat dyes (such as indigo), and naphthol dyes (which aren't used for hand dyeing here due to their dangers). All-purpose dyes, such as Rit, will work, too, since they contain direct dyes, but I don't recommend direct or all-purpose dyes for cotton or rayon, since fiber reactive dyes work so much better.
Like other forms of rayon, cupro is weak when wet, so care must be taken to avoid tearing or abrading it. I would recommend that, if you use a washing machine for dyeing cupro, you use only a delicate cycle, and that for machine laundering, later on, you consider placing the garment in a large mesh lingerie bag to protect it. I have successfully machine-washed many rayon garments, with only a couple of pieces becoming damaged, but there is always some risk of loss when washing clothing that is labeled dry clean only.
The best way to get a smooth solid color is to do the dyeing in the washing machine. (See "How can I dye clothing or fabric in the washing machine?".) Procion MX dye works extremely well for washing machine dyeing. It's best to use a top-loading washing machine for dyeing, though some have managed to do a good job in a front-loading washing machine, depending on the specific features of the washing machine in question, and in Europe you can even buy a prepackaged dye designed for use in a front-loader, Dylon Machine Dye, but you cannot buy Dylon Machine Dye in the US. Whatever dye and dyeing method you choose, it's important that the fabric is completely free of stains and surface finishes before you start.
Alternatively, you can get a large bucket to do the dyeing in, and follow a good recipe for immersion dyeing, the same as the washing machine dyeing recipe except for the smaller quantities, which is more trouble since it requires that you stir pretty much constantly for an hour. You will need Procion MX dye, soda ash, and ordinary non-iodized table salt. The bucket must be large enough for the piece of clothing to move freely when stirred. A pair of shorts should dye nicely in a five-gallon bucket, though something larger and heavier might require a larger container. I do recommend the far easier washing machine method if your washing machine will allow for it.
Dyeing in your washing machine will not harm it or stain any clothing that is later washed in it, as long as you wipe out any dye spatters above the water line with a damp cloth or paper towel, and do your after-dyeing washout of the excess unattached dye from your shorts in the same washing machine, allowing plenty of opportunity for the excess dye to be cleaned away. Some dyers like to run a load of towels with bleach immediately after dyeing in the machine, but I have not found this to be necessary. On one washing machine, I did see some staining of white sections with the turquoise dye only (Procion Turquoise MX-G, popular in itself and as an ingredient in many pre-mixed Procion dye colors), but this did not alter the color of other clothing that I later washed in the same machine.
A very important key in dyeing dark black is to use a lot more dye powder than you would use for a paler color. This is true for any class of dye and any type of fiber. You should use twice as much black dye for a rich deep black as you would use for a merely dark color, and you should use twice as much dye powder for a dark shade as you would use for a medium shade, so a good general rule of thumb is to use four times as much dye, when you are dyeing anything black. (See "How much Procion MX dye should I use?".)
Another important detail is that the thread with which your shorts were sewn together is almost certainly made of polyester, which means that it will remain undyed. Look carefully at your shorts to decide whether they will look okay in black, if the stitching at the seams is still the original caramel color. Although it is possible to dye polyester, doing so requires special dyes, smelly chemicals, and an hour's worth of cooking on top of the stove, using a dyeing pot you don't plan to reuse for food, so it's really not worth trying to do so.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, August 11, 2011
I want to dye basketball socks, orange on a royal blue sock. Can I dye the whole sock, or do I have to spot dye just the white areas?
Name: George
Country or region: Texas
Message: I want to dye some Nike ELITE basketball socks. My son wants the white parts to be orange on a royal blue sock. Can I dye the whole sock, or do I have to spot dye just white areas. Thanks and appreciate the advice.
This is not nearly as simple an idea as it seems to be at first.
First, Nike Elite basketball socks are treated with a surface finish called Dry-Fit. Surface finishes will tend to prevent any dye from penetrating to the fiber underneath, thus interfering with the dyeing process. They may also interfere with fabric paint, making it adhere less tightly to the fiber than you would like.
Next, and much worse, their fiber content is impossible to dye. Nike Elite basketball socks are composed of a blend of 62% polyester, 21% nylon, 15% cotton, and 2% spandex. The problem here is the combination of polyester with spandex. Spandex is an extremely heat-sensitive fiber and should never be subjected to temperatures over 140°F (more ideally, no temperatures over 105°F); at higher temperatures, it is likely to lose its shape and therefore its ability to stretch and contract appropriately. In contrast, polyester cannot be dyed at temperatures anywhere near as low as 140°F; the minimum temperature for dyeing polyester is far higher, at least 190°F, and a full boiling temperature of 212°, or even higher under pressure, works better. Because their dyeing needs are in conflict, the fibers in the polyester/spandex blends you see are always dyed separately, before they are blended together. (See "How to Dye Spandex".)
So, the conclusion is, you will not be able to dye these socks at all. Will you be able to color them another way? You might be able to use a fabric paint that is supposed to work on polyester. It will wear off more quickly than a true dye, if you had a dyeable fiber blend, but your fiber blend is not dyeable, so fabric paint is your only option.
Which brings us back to the question you thought you were asking, which is whether you can apply orange on top of royal blue. All dyes, and most fabric paints, are transparent, so the original color shows right through any color you apply on top of it. Since orange is opposite blue on the color wheel, orange dye applied on top of royal blue fabric will create a dull, dark brown. That's not what you're looking for. Although opaque fabric paints do exist, which are capable of covering the original color, they require a heavier, thicker application, so that the dry paints will feel scratchier on the fabric, and they will tend to show wear more visibly. Conclusion: if you must change the color of Nike Elite basketball socks, you should apply your orange fabric paint on the white sections only. By the way, you probably should not expect the fabric paint to last through the entire life of the socks.
Not all fabric paints will work on polyester. The company Jacquard Products, which makes several different types of fabric paint, including Dye-Na-Flow, a fabric paint that is designed to be almost as thin as dye, says that their fabric paints will all work on polyester, so I recommend looking for them. We don't know how well any fabric paint will be able to adhere to the surface finishes on the Nike Elite basketball socks, so I recommend that you try coloring only one pair, and watch through several wash cycles to see how well it wears. It's important to always test whatever materials you are using before investing a lot of time or materials into the effort. I strongly recommend that you always turn the socks inside out for laundering; line-drying instead of machine-drying will reduce the amount of abrasion the socks are exposed to.
You will get far better results, in dyeing socks, if you choose socks that are made of a natural fiber, or at least of fibers whose dyeing characteristics are not as incompatible as polyester and spandex. Cotton/spandex blend socks, for example, or rayon/spandex, are much better for dyeing, because both cotton and rayon can be dyed with a cool water fiber reactive dye, which does no harm at all to the spandex in the blend. I like the bamboo rayon socks that are sold by Dharma Trading Company. Polyester socks could be dyed by boiling them with disperse dyes, but only if there is no spandex in the blend to be damaged by the heat.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Monday, August 08, 2011
Is it possible to mix some of the Jacquard Red label dyes into the Green label to get slightly more intense colors, without steam-setting the dye afterwards?
Name: Imzadi
Country or region: USA
Message: I read where you said the Jacquard Red Label dyes are twice as concentrated as the Green Label. Is it possible to mix some of the Jacquard Red label dyes into the Green label to get slightly more intense colors? I prefer to use the dyeset instead of steaming.
I do have several old scarves made with the Red Label & old version of dyeset, BEFORE the Green label was even invented. The colors are so much more intense & luminous.
What if I do a vinegar soak before painting on a Green + Red label mixture?
No, you can't do this, not if you are going to continue to skip the steaming process. The manufacturer's instructions are very clear. For example, Dharma Trading Company, a retailer for many dyes including the Jacquard Red and Green Label Silk Colors, spells it out in detail:
"Red Label Silk Colors can be set only by steaming! They can not be set with Jacquard Dyeset Fixative concentrate like the Green Label silk color. The difference between Red label and Green label Jacquard Silk Color is depth of color. Red label dyes produce much more vivid colors because they are much more concentrated. But they MUST BE STEAM SET!"
I am curious as to what you mean by "the old version of dyeset". Do you think it was the same as the current Jacquard Permanent Dyeset Concentrate? How long ago do you think this was? The Rupert Gibbon & Spider brand Green Label Silk Colors have been available for many years. They've certainly been around longer than the DyersLIST mailing list, which referenced them in October 1996. Their formulas, or the formula of the DyeSet Concentrate, may have changed since they were first introduced, though, for all I know.
The Green Label Silk Colors are frequently said to give better, more intense colors when they are set by steaming, rather than by the Permanent Dyeset Concentrate. Steaming is a much better way to set the Green Label dyes on silk, if results are more important than convenience. If you want more intense colors, I recommend that you give up using the dyeset, convenient though it is. (For more information on steam-setting silk dye, see my page, "How to Dye Silk"; scroll down to "How to Fix Your Silk Dye" , to find instructions for steaming with ordinary kitchen equipment and other methods.)
Interestingly, you have more options in dyeing with the Red Label Silk Colors than you do with the Green Label Silk Colors. Unlike the Green Label Silk Colors, the Red Label version can be used at a high pH, instead of a low (acid) pH, if you wish, thus making steam-setting unnecessary, though a little heat is required. Both the Red Label and the Green Label dyes contain a type of fiber reactive dye known as Vinyl Sulfone dye, also known as Remazol dye, for the first brand name under which it was sold. The Green Label Silk Colors are acidified, with vinegar or another acid, in addition to having had some of the Permanent Dyeset Concentrate or similar product mixed in with the dyes. This means that they cannot be used under high-pH conditions, since the acid included in the formula will neutralize a high-pH chemical such as soda ash.
When you use the Red Label Silk Colors with vinegar, or another acid, you must use heat to set the dye, and you cannot use cellulose fibers such as cotton. Using the Red Label Silk Colors with vinegar means that you must steam-set the dyes on your fabric, in order to bond them permanently to the fiber.
However, the Red Label Silk Colors contain pretty much just dye and water, so they can be used with soda ash instead of vinegar. When the Red Label Silk Colors are used with soda ash instead of vinegar, a dye/fiber reaction temperature of 104°F to 140°F works fine, given the right recipe, on both silk and cotton, without any need for either steaming or the use of Dyeset Concentrate. Dyes can be mixed with sodium carbonate and urea (to maintain moisture), or applied to fabric that has been soaked in sodium carbonate and then line-dried, though it's essential that the cup of dye into which you put your paintbrush contain only enough dye for a few hours' use, since the paintbrush will contaminate the dye cup with soda ash, even if the soda ash is dry in the fabric. See "Vinyl Sulfone Fiber Reactive Dyes".
I no longer recommend buying Red Label Silk Colors, because now there are other lines of Remazol type dyes available. Jacquard's newer line, called Vinyl Sulphon dyes, contains the same type of dyes as Jacquard Red Label and Jacquard Green Label, but they are much more concentrated than either, and therefore far more economical to buy. Jacquard's Vinyl Sulphon dyes are at least four times as concentrated as Jacquard's Red Label Silk Colors. Very similar in both price and quality to Jacquard's Vinyl Sulphon line is the Liquid Fiber Reactive line of dyes sold by PRO Chemical & Dye; I happen to prefer ProChem's black dye to Jacquard's, since it is a single-color unmixed pure dye, and, moreover, has better lightfastness, but the other dyes in the two lines are mostly very similar, as are the dye concentrations. I can't see any reason to buy Jacquard Red Label Silk Colors now, when the Jacquard Vinyl Sulphon dyes contain the same dyes in a much more economical form.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, August 04, 2011
How can you tell to which class of acid dye a particular dye belongs?
Name: Zoe
—ADVERTISEMENTS—
Books about the chemistry of dyeing
Wool Dyeing
Edited by David M. Lewis, 1992.
Highly technical and thorough, this book explains all aspects of wool dyeing in a manner understandable by textile professionals and advanced science students.
Victor B. Ivanov's

Reactive Dyes in Biology and Medicine
Explains use of reactive dyes for staining proteins or carbohydrates
Waring and Hallas's

The Chemistry and Application of Dyes (Topics in Applied Chemistry)
includes recipes for synthesizing reactive dyes
Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
John Shore's

Cellulosies Dyeing
Useful information about the chemistry of reactive dyes, and other dye types used for cotton and other plant fibers
Country or region: Switzerland
Message: I would just like to ask a questions about the different types of acidic dyes: How do you tell that a dye is of which type? The dye in particular I am enquiring about specifically is Acid Blue 93. I think it is a acid-milling dye, but I am not sure. Thank you!
This is an interesting question. How can you tell to which class of acid dye a particular dye belongs? This question cannot be answered briefly.
The different acid dyes, not including the metal complex dyes, are classified into four different groups, rather arbitrarily, based not on any structural consideration, but instead on their wet fastness properties. From least washfast to most washfast, they are categorized as:
1. acid leveling dyes (also known as level-dyeing, strong acid, or equalizing acid dyes)
2. fast acid, half-milling, or perspiration-fast dyes
3. acid milling dyes
4. supermilling dyes
(From the 1992 book, Wool Dyeing, edited by David M. Lewis, in a chapter entitled "Dyeing wool with acid and chrome dyes", by P.A. Duffield.)
Acid milling dyes are named for possessing more wet fastness on textiles, during milling treatments, than some other groups of acid dyes. The optimal pH for their attachment to textile fibers is much higher than the pHs required for levelling dyes. While acid levelling dyes work best at a pH between 2.5 and 3.5, acid milling dyes work best at a much more mildly acid pH of 5, ranging all the way up to the very mildly basic pH of 8.
Experimental comparisons of wet fastness and optimal pH would be a slow and lengthy way to identify the classification of your acid dye. However, in this case, we're in luck: the molecular structure for Colour Index Acid Blue 93 is readily available online, on multiple sites. Let's see what we can make of it from its structure. Colour Index Acid Blue 93 is also known as Methyl Blue (not to be confused with Methylene Blue), Helvetia Blue, Ink Blue G, and Cotton blue, among others:
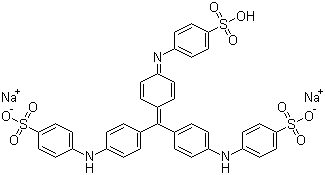
(from Chemblink.com, at http://www.chemblink.com/products/28983-56-4.htm)
It is also known as Colour Index number 42780, or, to give the full chemical name, as disodium [[4-[bis[4-[(sulphonatophenyl)amino] phenyl]methylene] cyclohexa-2,5-dien-1-ylidene] amino]benzenesulphonate. Its molecular weight is 799.8, and its CAS registry number (often useful in performing further searches) is 28983-56-4.
To start with, we can tell by looking at the structure that this is an acid dye, which means that it should be suitable for dyeing polyamide-based textile fibers, such as silk, wool, and nylon. (In spite of their name, acid dyes are not themselves strong acids, but instead are named for the usually mild acid used as an auxiliary when dyeing textiles with them.) We can see that this dye structure is not a fiber reactive dye, because there are no reactive components, such as a dichlorotriazine section. It's not a basic (cationic) dye, because, without the associated sodium ions, it has a negative charge; basic dyes have positive molecular charges. It's not a vat dye, both from the obvious fact that it's a salt, rather than being neutral in charge, and because it lacks ketone (C=O) groups, which are part of the structure characteristic of vat dyes. Unlike a direct dye, it is not long, narrow, and planar in shape; the aryl groups are twisted, instead, creating a propeller shape (as cited by Dean Thetford of Zeneca Specialities in 2000 in a piece entitled "Triphenylmethane and Related Dyes" that appeared online in the Kirk-Othmer Encyclopedia of Chemical Technology and in Van Nostrand's Encyclopedia of Chemistry). There are no carboxylic groups adjacent to hydroxyl groups, as you may see in mordant dyes. It's obviously not a metal complex dye (also known as a pre-metalized dye), because there are no heavy metals indicated in the structure at all. Like other acid dyes, it contains sulfonate groups, which help to make it water-soluble. It's interesting to note that some dyes that belong to other classes, such as fiber reactive dyes, include these sulfonate groups, too, and are therefore, under the correct circumstances, capable of acting as acid dyes. (See "What kinds of chemical bonds attach dyes to fibers?".)
Looking at its chemical structure, the chromophore (color producing section) of this dye is clearly of the triarylmethane type, resembling the chemical triphenylmethane. Acid dyes having structures related to triphenylmethane are said to predominate in the milling class of dye, as mentioned in Wikipedia since 2006, but I don't see a proper reference for this claim there; much more recently, this statement has appeared, word-for-word but without proper attribution, in Dharma Trading Company's useful new page on how acid dyes work. I'm not quite sure whether the statement is intended to claim that most triphenylmethane dyes as acid milling dyes, or that most acid milling dyes are triphenylmethanes; this particular Wikipedia article is not written to as high a standard as many other Wikipedia entries. A number of dyes with similar triarylmethane chromophores, but lacking the sulfonate groups, are found among the basic dyes, including some of the first dyes synthesized, such as crystal violet. Triphenylmethane dyes are noted for poor lightfastness.
That said, the surest way I know to determine whether an acid dye is in the group known as acid milling dyes is to find it listed as such for sale. I have not myself been able to find Colour Index Acid Blue 93 listed as an acid milling dye, or as a leveling acid dye, or any other specific category of acid dye. It does not appear to be much used in textiles, but instead appears to be manufactured mainly for use in inks, in addition to its use as a biological stain. Many acid milling dyes are listed as such by their manufacturers; for example, Town End Colours for Industry has a page listing Acid Milling Dyes for Nylon and Other Polyamides, not including C. I. Acid Blue 93. It's not included in the listing of acid milling dyes for any manufacturer I have looked at. Although PRO Chemical & Dye's and Maiwa's lines of WashFast Acid Dyes (formerly made by Ciba) comprise mostly fast milling dyes, it is not included among them, either (see my page, "Which Washfast Acid colors are pure, and not mixtures?"), nor is it included among the Jacquard Acid Dyes. In fact, Acid Blue 93 does not appear to be available as a textile dye from any of the suppliers of textile dyes for hand dyers. It looks as though it is best obtained from a supplier of biological stains, such as Sigma Aldritch.
(Please help support this web site. Thank you.)
There's no obvious structural difference between a leveling acid dye versus an acid milling dye, since acid dyes are classified according to fastness rather than by structure, but the size of the molecule is itself a good indication. Smaller molecules are better at leveling, but are less wet fast; larger acid dye molecules are worse at leveling and better at wet fastness. The relative molecular weight of this dye is typical of an acid milling dye. Its relative molecular weight is listed in various online sources as 799.8. According to David M. Lewis's book, Wool Dyeing, the relative molecular weights of acid milling dyes range from 600 to 900. In contrast, the relative molecular weights of acid leveling dyes, also known as strong acid dyes, range from 300 to 500 for the monosulfonated dyes, and 400 to 600 for the disulfonated ones. (Almost all levelling acid dyes contain either one or two sulfonate groups.) The fast acid dyes, also known as half-milling or perspiration-fast dyes, range from 500 to 600 relative molecular mass and are typically monosulfonated. So, just looking at the size of the molecule, we can be pretty sure that Acid Blue 93 is an acid milling dye, though we can't distinguish it from a supermilling dye.
(Please help support this web site. Thank you.)
Tuesday, August 02, 2011
Will Retayne set a dye originally intended to be washed out?
Name: Cynthia
Country or region: Nebraska, USA
Message: Will Retayne set a dye originally intended to be washed out? I bought an old feed sack (off-white with colored logo) with printed instructions for washing out the logo. The instructions are to wash in water greater than 150 degrees. I want to retain the logo and use fabric in a quilt that will be rarely washed. How can I prepare and care for this fabric to preserve the print?
I can't recommend using Retayne to try to make any water-soluble dye permanent. Retayne, and other brands of cationic dye fixatives, are unlikely to be able to set this type of dye, even sufficiently for an item that is to be washed only rarely. The biggest risk is that the logo may run while you are trying to apply the Retayne.
Retayne is supposed to be applied in hot water. I am afraid that, even if you use water that is 130°F or 140°F to apply the Retayne, at least some of the ink in the logo may dissolve in it. Although the instructions for your water-soluble dye say to use 150°F water to wash it out, it is very likely that some of it will run even in cooler water.
Here are several better ways to make the logo permanent:
1. Never, ever wash the feed sack. Frame it behind glass.
2. Using fabric paint selected to match the logo's color(s), carefully paint over the design. Allow the fabric paint to dry and then, if the manufacturer recommends doing so, use a hot iron to heat-set it. (Some fabric paints do not require heat-setting, but it is always best to wait at least a week after they dry before washing.)
3. Scan the logo on your computer's scanner, or have a photocopy shop do this. Print it, or have the photocopy shop print it, onto iron-on transfer paper, or have the photocopy shop transfer it to fabric for you.
4. Using a clear, colorless fabric paint, generously paint over the logo on both the front and the back sides of the fabric, using the clear acrylic paint to seal in the water-soluble ink. Note that this, like colored fabric paint, will change the feeling of the fabric, at least a little, even if it does not change the color. PROfab Transparent Base Extender (which is sold online by PRO Chemical & Dye) would be a good choice. Other products that should work well for this include Jacquard Products' Neopaque Extender, or an acrylic textile medium such as Jo Sonja's Textile Medium or Delta Ceramcoat Clear Textile Medium. Acrylic textile mediums are sold for the purpose of mixing with artists' acrylic paints for making your own fabric paint. The different brands of products have different textures when wet; please experiment on scrap material before using any of them on your project.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Monday, August 01, 2011
Is there a dye pen I could use to "color" over the embroidery?
Name: Shari
Country or region: USA
Message: Hi, I own a beautifully embroidered pillow that I would like to change a color on. Part of the embroidery is a rusty orange which completely clashes with the red in my room. Is there a dye pen I could use to "color" over the rusty orange, making it more in the red family? What do you recommend? Thank you for your help!
Yes, you can do this, by carefully drawing on the embroidery using fabric markers, which contain pigments rather than true dyes. You can color on top of a clear orange to make it a true red. A rusty orange will produce more of a brownish red, but it should be a closer match than what you have now. The only real worry is whether it will be easy to confine the color to the thread you're trying to darken, or whether it will be difficult to keep from splotching color onto the background, as well.
You'll want to start by testing your fabric marker. You should get some scrap fabric, and maybe make a few stitches of embroidery in it, to use for testing. Unfortunately, you probably have no idea what the fiber of the embroidery thread is made from, so you won't be able to be sure that it will take it as well as your test thread does. Synthetic fibers may take the color from a fabric marker more lightly than a natural-fiber yarn would do. At least you can check to make sure that it's not too difficult to keep your marker on the line of stitching, though.
You will probably want a fine-tip marker. There's a wide variety of fabric markers available. I do not recommend any marker that doesn't specifically say it's for use on fabrics. In my experience, standard Sharpie pens, for example, wash out much more quickly than fabric markers do. (I believe that Sharpie has introduced a new line of fabric markers, called "Stained By Sharpie"; these are probably good, but I have not tried them or seen any reviews of them yet.) Check the label carefully, to make sure whether you need to heat-set the color afterwards, by pressing it with a hot iron; some fabric paints and markers require heat-setting to become permanent.
You will probably be able to find good fabric markers in your local crafts store. If you don't, take a look at the wide range of fabric markers sold by Dharma Trading Company.
(Please help support this web site. Thank you.)


