« 2012 June | Main | 2012 April »
Thursday, May 31, 2012
Can I dye small areas of my riding vest from red to green?
Name: Norman
—ADVERTISEMENTS—

 Neopaque is an opaque fabric paint, so white and light colors can cover a dark or colored background.
Neopaque is an opaque fabric paint, so white and light colors can cover a dark or colored background.
Country or region: Florida, US
 Message: I am trying to dye small areas of a riding vest I wear on my bike. The areas are currently red and I want to dye them like an OD Green (dark green). I looked up to see if I could find out what kind of fabrics it is, the only thing I seemed to find was that it may be Tri-Tex Fabric and Mesh. I have the one that is black and red.
Message: I am trying to dye small areas of a riding vest I wear on my bike. The areas are currently red and I want to dye them like an OD Green (dark green). I looked up to see if I could find out what kind of fabrics it is, the only thing I seemed to find was that it may be Tri-Tex Fabric and Mesh. I have the one that is black and red.
Thanks you very much for your time!
The only thing that will work to turn red to green is an opaque fabric paint.
No dye will change red to green; if you try to dye something that is red to green, you will end up with brown or black, because red and green combine to make brown or black.
As you discovered, it's hard to determine the exact fiber content of that vest, but it looks like a synthetic of the sort that won't take ordinary dyes anyway. The dye that works best on synthetics is disperse dye, but you have to boil the item in the dye, or make an iron-on dye transfer. I'd be afraid to boil that vest, for fear parts of it might shrink or melt. But, again, you can't use dye to turn red to green, so you wouldn't want to try it anyway.
The only problem with using a fabric paint for this is that paint might not stick to a slick shiny material. Buy a small jar of opaque fabric paint and test it somewhere on the inside, so that you won't have ruined anything if it doesn't work well. If the material is very slick and shiny, consider whether or not roughing it up with a bit of sandpaper might help paint to stick.
If the material feels like fabric, then fabric paint is the right product to use. If it is stiff and hard, then another type of paint might work just as well, as long as it is opaque enough to cover up the red color. Fabric paint is better for sticking to fabric, and for feeling soft after it dries, but that won't matter much if the material is stiff to begin with, instead of soft like fabric.
You might be able to find opaque fabric paint at your local crafts or sewing store, or you may have to order online. For a plain opaque color, Jacquard Products makes Neopaque brand fabric paint, but you'll probably have to mix a little black into the green to get an olive drab color; they also have a metallic olive green in their Lumiere line of metallic fabric paints.
(Please help support this web site. Thank you.)
Wednesday, May 30, 2012
Is it possible to use oil-based paints on fabric, thinned and sprayed with an airbrush?
Name: Glen
Country or region: Australia
Message: Is it possible to use oil-based paints on fabric, thinned and sprayed with an airbrush?
There are a couple of problems with using oil paint instead of fabric paint or fiber reactive dye.
One of the problems is that oil paint will be much stiffer, after it dries, than a good soft fabric paint. It will feel scratchy and rough, even though it was diluted before application.
The other issue is the question of how much damage the linseed oil in the oil paint will do to the fabric. This doesn't matter for a short-lived project, such as a temporary costume that you don't plan to keep (or sell), but it does matter for anything that ends up being worth keeping for years, and for any work that you sell.
Oil painting is traditionally done on linen or cotton canvas that has been primed with many layers of rabbit skin glue; now we usually use an acrylic-based gesso for the same purpose. This protective layer prevents the caustic effects of linseed oil from damaging the canvas. Air-brushing thinned oil paint directly onto fabric bypasses this protective layer.
Fabric paints are more suitable for air-brushing fabric, if you want your results to feel nice enough to use in clothing, or if you want to use materials that won't eventually damage your fabric. Since fabric paints are water-based, they should be thinned for airbrushing with water, rather than solvents; this reduces risks to the painter, as well, since it's dangerous to inhale solvents. I recall an art professor's becoming gravely ill with aplastic anemia, when I was in college, as the result of solvent exposure. Even without the solvents, however, it is still very important to wear a dust mask or respirator to avoid inhaling any particles of paint.
Jacquard Products recommends that you thin their fabric paints, such as Dye-Na-Flow or Lumiere, with up to 25% their volume in water, if you are using them for airbrushing. They also manufactures a product called Jacquard Airbrush Ink, which is another acrylic-based fabric paint.
Another option for air-brushing fabric is using fiber reactive dyes, such as Procion MX dyes. The dyes are dissolved in water, filtered through a bit of nylon stocking to be sure there are no undissolved particles, and fixed on the cotton fabric with dissolved soda ash; the soda ash can be used as a pre-soak for the fabric, or, if you're working fairly quickly, mixed with the dye itself. The result, after excess dye is washed out, is a permanent painting that cannot be felt at all on the fabric. Aesthetically, the results of air-brushing fiber reactive dye is much better than the use of paints, since it leaves the hand of the fabric completely soft. However, a difficulty is that not all of the dye bonds to the fabric, so it is necessary to apply it to a darker color intensity than is ultimately desired.
For good sources for ordering Jacquard Airbrush Inks or other fabric paints, as well as Procion MX dyes and other fiber reactive dyes, see my page, "Sources for Dyeing Supplies Around the World"; scroll down to the section on Australia.
(Please help support this web site. Thank you.)
Tuesday, May 29, 2012
Is there a way to tie dye a tent?
Name: Bill
DWR finishes are incompatible with dyeing. DWR finishes should be applied only after dyeing is completed.
Country or region: USA
Message: Is there a way to tie dye a tent? Thank you.
You can't dye or paint any commercially-available tent, because the DWR (durable water resistant) coating will prevent any dye or paint from reaching the actual fibers. Water beads up on the surface, and so will water-based liquids such as dyes and fabric paints.
However, you can have a tie-dyed tent! What you will need to do is to buy some untreated thin fabric and use the fly from a tent as a pattern to construct your own non-water-resistant tent fly, which you can waterproof after dyeing. (If you're not familiar with modern tent construction, the fly is a separate piece that attaches to the top of a tent to provide protection against rain.) Use your tie-dyed tent fly in place of the one that was sold with the tent; the overall effect will be of a tie-dyed tent, since the fly is by far the most visible part of your tent. Alternatively, you could make a very simple rectangular tent fly, staked at each corner, with a pole and guy line at each end, to mount over your tent. Choose the color of the main structure of your tent so that it will go with your tie-dyed fly.
You will have to test-dye a small snippet of the fabric you choose for your new tent fly, to make sure that it will accept the dye or fabric paint, before you begin to sew. Ask your fabric supplier to give or sell you a small swatch of each of their likely fabrics for this purpose. Be sure to tell them that you want untreated fabric, not water-resistant fabric. If necessary, test the fabric by sprinkling a few drops of water on it, to make sure that they soak in immediately, instead of beading up; don't even try to dye a fabric that is water-resistant. If you don't have a local source for a suitable material, try one online, such as a silk or nylon fabric from Dharma Trading Company, or a non-water-resistant ripstop nylon from The Rain Shed, if they sell one that is not treated. The Rain Shed sells other parts that you may need, as well, including webbing, hooks, and zippers.
Try to find material that is labeled as PFD ("Prepared For Dyeing") or PFP ("Prepared For Printing"), which lack surface finishes that will interfere with dyeing. Dharma's nylon PFD fabric is guaranteed to be dyeable, so it's an excellent choice; nylon from other sources often has invisible treatments that interfere with dye. In addition, pre-wash your material before beginning dyeing, using very hot water, Synthrapol detergent, and extra soda ash to try to remove any remaining fabric sizings or finishes.
Once you have constructed your new non-water-resistant tent fly, or perhaps working with the fabric before constructing the fly from it, you can tie-dye it. You will have to choose your dyes and methods according to the fiber content of the fabric you chose for your fly. Silk is extremely easy to dye with many different types of dye, including an ordinary tie-dyeing kit such as the excellent ones made by Jacquard Products, but it is less durable. Nylon can be dyed with a type of dye called acid dye; it requires heat to set it, which can be arranged by steaming the fabric, in a way similar to steaming vegetables, after applying the dye and letting it dry. Polyester is more difficult to dye and requires disperse dyes, but there are a couple of very different methods for applying it. A good thin fabric paint is another alternative for tie-dyeing untreated nylon or polyester. Avoid non-dyeable fabrics such as polypropylene or polyester/spandex blends.
After you have completely finished dyeing your tent fly, you will need to apply a durable water repellent finish. You can find this type of product from a supplier for camping or mountaineering, since DWR finishes on tents and outdoor clothing tend to wear off and must be replenished at least once every ten years. My first choice would be Nikwax TX-Direct Wash-In, which can be applied in a washing machine; I think the wash-in product is more suitable as the first waterproofing treatment for a new item that has not been waterproofed before. Spray-on DWR finishes are also available.
(Please help support this web site. Thank you.)
Monday, May 28, 2012
I opened a couple of my jars of dye to take a look at it and my colors don't look anything like the color chart samples they show you on their website.
Name: Louise
Country or region: USA
Message: Hello Paula,
I recently purchased Dharma fiber reactive dye from dharmatrading.com. I've never used Procion dye before, only Rit, that being the reason I switched to fiber reactive. I'm a little concerned. I opened a couple of my jars of dye to take a look at it and my colors don't look anything like the color chart samples they show you on their website. The rust brown looks more of a red than a brown, and the grayish brown I ordered looks like a basic brown. I'm wondering if maybe they put the wrong colored dye in the jars. In your experience, is this normal? Will they look more like the colors I wanted after I add chemicals and the like, or did they give me the wrong dye? It's not a matter of hue or shade of the color, its more like the colors are the wrong type of that particular color.
Don't panic! There is nothing wrong. Good dye powder is always so much darker than the color it will produce that it often appears nearly black, and it can appear to be quite a different hue, as well, depending on the individual dye color.
The only reason that Rit dye may appear to be anything like its final color is that it is highly dilute. Each packet of Rit dye contains only a tiny quantity of dye, mixed together with a very large quantity of sodium chloride (table salt) and laundry detergent. One packet apparently contains only about 2 grams of direct dye plus about 4 grams of leveling acid dye, in addition to about 26 grams of relatively inert ingredients. Since the vast majority of the ingredients of the Rit package are inert white powders, the tiny amount of dye in the mixture is lightened to the point that it can resemble the final color produced.
In contrast, the Procion MX fiber reactive dye you've purchased is highly concentrated. It contains no table salt (the sodium chloride that makes up so much of the Rit mixture) and almost no detergent. It contains only small amounts of other ingredients for essential purposes, including stabilizing the dye, helping to prevent the dye from dusting into the air, and standardizing the colorizing power of the dye by weight so that one dye lot is the same strength as another. (Dye powders are standardized by weight, not by volume, so that one gram of dye is always the same strength; however, one package may be more or less dense than another, so measuring by the teaspoon is less accurate, though often good enough.)
There is no reason to expect any dye to be the same color in powdered form that it will be on the fabric. For example, a green or navy blue dye will normally appear almost black in the jar; a yellow dye will appear to be orange. Yellow dye that will produce a light yellow on your fabric looks orange even after you mix it with water. The greater amount of surface area of a powder scatters the light, making the material appear darker. There can be chemical changes in dye as well, when it is dissolved and allowed to bond the a textile fiber, which means that some colors look quite different, and not merely darker, when in dry powdered form. Fortunately, we don't care what color the dye powder may appear to be in the jar, as long as the dyed textile material turns out to be the right color, after the dye has bonded.
Your results with the Procion MX dye you ordered from Dharma Trading Company will be much better than what you could obtain with all-purpose dye. Fiber reactive dye, such as Procion MX dye, will stay bright for years instead of weeks, and, once you've washed out the excess unattached dye from the dyeing process, it absolutely will not run in the laundry. Although Rit-dyed clothing must be hand-washed separately from other garments, to avoid ruining items of different colors when the dye bleeds in the laundry, it is safe (after the first few washings) to dump Procion-dyed cottons into the same washing machine load as white clothing, and even wash them in hot water, without any color transfer occurring. If you dye all of your clothes with Procion dye, you can give up sorting your laundry by color.
Instead of using boiling water to set the dye (Rit works best in simmering water at a temperature of 190°F), you simply add soda ash or washing soda, to chemically set the dye, so there is no need to heat the dye with the fabric to get it to perform as well as it can. Washing soda is a major constituent of laundry detergent powder, so it's something you've probably used many times, though the pure form is what's needed here.
Procion MX dye is far more versatile than Rit dye in how it can be applied. Like Rit dye, it can be used in a large volume of water (adding salt, which is much cheaper purchased separately) to produce a single solid color; it can also be mixed with small amounts of water and, optionally, some thickener, to make dye paints for painting on fabric. It's mixed with small amounts of water and applied to fabric that has been presoaked in soda ash water for tie-dyeing. Because there are so many ways to use Procion dye, it's important to find a recipe for the method you wish to use first, and follow the recipe closely, until you are familiar with the way the dye works. There are good recipes on my website; there are also many recipes on the Dharma Trading Company web site. These are excellent resources for learning to use your dye.
(Please help support this web site. Thank you.)
Friday, May 25, 2012
I dyed a pair of shoes yesterday, and while they look wonderful, I simply cannot get all the dye out.
Name: Irwin
Country or region: USA
Message: Hi there, your site has been incredibly helpful!
I'm having an issue, though. I dyed a pair of shoes yesterday, and while they look wonderful, I simply cannot get all the dye out for the life of me. I cold rinsed them once, then used hot tap water and rinsed them each for 30 minutes. When that was still not running clear, I boiled water on the stove and poured 3 deep saucepans worth of boiling water through them, and they're *still* not running clear!
What do I do? After reading a bit on your site it seems like the hard water we have in the house is the reason, but I don't know what to do with this pair of shoes. The company doesn't exist anymore-- that's why I'm dyeing them, actually, because they only came in white!
If I have to I'll just sacrifice socks for the next two years and deal with it, but if you can suggest how I could get the dye out I'd greatly appreciate it.
In any case, thank you for your wonderful site, and have a great day!
Are the shoes' uppers made of a natural plant fiber, such as cotton, linen, or hemp? Those are the best materials to dye. Dyeing cotton canvas shoes can work wonderfully. Unfortunately, polyester and nylon require completely different dyes and application methods, while some other synthetic shoe materials cannot be dyed at all except during their manufacture.
Can you tell me what kind of dye you used?
If it was good fiber reactive dye, such as Procion MX dye, and if you were using it on cotton canvas shoes, then it's likely either that your shoes' fabric contained starch sizing, or that you're right that the hard water caused this problem in the first place. If it was all-purpose dye, well, that's just the nature of the dye; all-purpose dye tends to bleed a little bit forever, with some colors being worse than others. Starch used as a fabric sizing is very difficult to remove completely, but, as the starch takes the same dyes that cotton does, the gradual wash-out of the dyed starch particles looks just like the dye is bleeding forever, when it's not really the dye. Exceptionally hard water can make it harder to wash out Procion dyes, as the result of larger complexes that form between Procion dyes in the presence of the calcium ions from hard water.
In either case, you've been doing the exact right thing to try to remove the excess dye. Hot water works best. Sorry it has not been effective! You can try soaking them in hot water, the hotter the better, before washing again in hot water. I hope the shoes are sturdy!
Another question: did you fix the dye properly? Procion and other fiber reactive dyes need a high-pH chemical, such as soda ash, in order to bond to the natural fibers they work on.
One more thing you can try is soaking the shoes in hot water with a cationic dye fixative, which you'll have to order online unless you happen to have an excellent quilter's supply store nearby. This fixative will help with any dye that has a negative molecular charge, including fiber reactive dyes such as Procion or the direct dye included in the mixture that is called all-purpose dye, assuming that you choose the correct type of dye for the fiber that you have. (It won't work if your shoes are made of polyester or another difficult-to-dye synthetic fiber, and it won't work if you used only acid dyes on a plant fiber.) I recommend that you order either Retayne or Dharma Dye Fixative, as they are the most economical products of this type, and they also seem to be better, judging from their instructions, than the similar product Rit Dye Fixative.
Thursday, May 24, 2012
My dress seems to have conflicting problems. It is 52% cotton, 46% nylon, and 2% spandex. Can it be dyed?
Name: Alyce
Country or region: USA
Message: I found a perfect dress for my daughter's beach wedding, but it is ivory - can't compete with the bride! It needs to be some shade of brown. I read all your info but my dress seems to have conflicting problems. It is 52% cotton, 46% nylon, and 2% spandex. Can it be dyed? Not hot, b/c of spandex. Not cold b/c of cotton? Split the difference? Don't try to dye it? HELP!
First, is this dress washable? You can't dye it if you can't wash it. You must prewash thoroughly before dyeing, and wash a lot immediately after dyeing, as well. See my page, "Can I dye clothing that is labeled 'dry clean only'?".
Assuming it's washable, my choice for this dress would be a cool water fiber reactive dye, such as Procion MX dye, because it won't require heat that may damage the spandex or shrink the cotton. It will do a great job of permanently dyeing the cotton, and will leave the nylon and spandex white. The overall effect will be of a solid color that is paler and more pastel than you would get on 100% cotton, half as intense in color. This is an excellent choice if you would like to dye your dress a light to medium color, but it will not work for dyeing it a dark intense color, such as black or brilliant red. A light brown is very doable, using Procion MX dye on a dress with this fiber content, but a dark brown is not.
You can use a hot water all-purpose dye, such as Rit, if you care more about dyeing both the cotton and the nylon at the same time than you do about being careful with the spandex or about shrinking the cotton; that's the choice you have to make. I think shrinkage is more of an issue than the spandex, since it's only 2% spandex. Using all-purpose dye on this dress is a reasonable choice if you need to obtain a dark or intense color, which requires dyeing both the cotton and the nylon. All-purpose dye is poorly washfast and fades pretty quickly, but it's the easiest way to dye this dress a dark color. You'll need to add a cup of vinegar to the dye-water so that the nylon will dye. In spite of the apparent promise of the "all purpose" description of the dye, the polyester stitching that holds the dress together at the seams and hems will still stay white, which may or may not be a problem, depending on the design of the dress; the same is true with Procion dye, which does not work on polyester.
For step-by-step directions for dyeing a wool- or nylon-blend garment with all-purpose dye, see my blog entry from May 11 of this year, "How to dye a wool blend coat with Rit All-Purpose dye". However, dyeing nylon is easier than dyeing wool since you don't need to worry about felting, so you can dye your dress in the washing machine, if you prefer, instead of having to stir it by hand for an hour. You can use the washing machine with either all-purpose dye or with Procion dye.
I like dyeing cotton with a cool water dye because you have much less need to worry about shrinkage, there's no risk of damaging the spandex, and it's also less trouble to set the dye in a garment by adding washing soda or soda ash, as you do for Procion dyes, than it is to have to use very hot water. All-purpose dye works best when simmered on the stove, around 190°F, though it's possible to compromise by using 140°F water, still scalding hot. Procion MX dye works perfectly well at room temperature, 70°F or above, so you can use it in a plastic bucket without any problem. Another advantage to Procion dye-clothing is that the color lasts nearly forever, and is safe to wash with your regular laundry (after you've washed out all the unattached excess dye leftover from the dyeing process); Rit all-purpose dye fades badly, and the dye tends to bleed and ruin other clothing in the laundry.
For the best color choice, order your Procion dye online, from a company such as Dharma Trading Company, PRO Chemical & Dye, or Blick Art Materials. You'll also need soda ash or washing soda, and, if you're dyeing a single solid color in a bucket or washing machine, you will also need a lot of salt. Do not use vinegar in this project if you use Procion dye, since it will neutralize the soda ash; use vinegar only if you're dyeing with all-purpose dyes or acid dyes on nylon or wool.
(Please help support this web site. Thank you.)
Wednesday, May 23, 2012
We are looking at buying 2 antique couches that appear to be velvet. They would be very expensive to reupholster, so we would have to dye them a bold red to work in our project. Which dye would you recommend for them?
Country or region: USA
Message: Hi there.
Thank you for your helpful link here on dyeing:). We are looking at buying 2 antique couches that appear to be velvet. They would be very expensive to reupholster, so we would have to dye them a bold red to work in our project. Im not sure how to tell if they are synthetic or whatnot, I was thinking since they are probably about 100 years old that the type of fabric they used would probably be natural. ??? If so, which dye would you recommend for them?
Thank you so much for your time.
I don't recommend using any dye on upholstered furniture, unless you are able to remove the fabric completely from the furniture in order to dye it. (See "Can furniture be dyed successfully?", in my FAQ list.) Dyeing requires a lot of washing to remove the excess unattached dye, which is impossible to do well if you don't remove the fabric. (See this post in my dye blog for a cautionary tale of what happens if you don't rinse out the excess dye!)
It is, however, possible to use fabric paint on upholstered furniture, especially if the fabric is made from natural fibers. The results will not last as long as new upholstery would, because fabric paint sits on the surface of the fabric and eventually wears off. Also, it can take a large quantity of fabric paint to cover a couch, which can drive up the cost to the point where it would make more sense to get a book on doing your own upholstery and try to reupholster it yourself.
What color are the couches now? Whenever you use dye or thin fabric paint to recolor anything, the new color will combine with the old color as though you mixed them together. You cannot use fabric paint to substantially lighten the color of fabric. Choose your color of fabric paint so that it is able to cover what you have now. A bold red is a good choice for light beige or pale yellow couches, but won't work on a brown or a green couch.
Don't try to buy your fabric paint at the local crafts store; they sell only tiny jars, so the price is much higher. Instead, order gallons of fabric paint online from Dharma Trading Company, PRO Chemical & Dye, or Jacquard Products. Although a 2.25 ounce jar of, say, Dye-Na-Flow fabric paint will run you over $4 at the local crafts store, totaling an extravagant $249 per gallon of fabric paint if you buy enough of the little jars, you can buy a full gallon of the identical product in a gallon jug from Dharma for only $56, one-tenth the price. This makes a huge difference in the practicality of this project for someone who can't afford reupholstery.
Don't consider using any other kind of paint besides fabric paint, such as house paint or ordinary artists' acrylics, because it will be horribly stiff and scratchy. Fabric paint is formulated to be softer and stick to the fabric better. You can turn artists' acrylics into fabric paint by mixing them with fabric medium, but I recommend buy a good thin fabric paint in bulk, instead, such as Setacolor or Dye-Na-Flow. You can follow Deborah P. Harowitz's excellent "Instructions for Painting Upholstered Furniture". Read through these instructions now to get a better idea of what is involved.
For similar results, consider a product called Simply Spray Upholstery paint. The spray fabric paint concept is a clever one. You might try spraying with both bright red and burgundy upholstery fabric paint. One can of Simply Spray Upholstery paint should cover up to 17 square feet, or an area roughly four feet by four feet, so you'll need many cans. Of course any non-fabric elements in the couch design must be very carefully covered to protect them from splatters or smears of either sort of fabric paint.
Do keep in mind, however, that many do-it-yourselfers have succeeded in home reupholstery or making well-fitted custom slipcovers. These are certainly options you should consider as alternatives to painting your couches.
For more blog entries on the subject of dyeing couches, please see:
(Please help support this web site. Thank you.)
Tuesday, May 22, 2012
Name: Rochelle
Country or region: NY, US
Message: In your blog, you write that Rit Fixative should be used in a separate dye bath after dyeing, because the OP had already dyed her fabric. Is it possible to skip a step and pour the fixative into the dye bath, thus draining excess dye from the fabric only once?
It's not a good idea, for more than one reason. The first reason is that, if you're planning ahead this much when dyeing, you probably should not even be using an all-purpose dye, such as Rit, at all. The only time it's really a good idea to use all-purpose dye is when you're dyeing a garment that contains a blend of both nylon (or wool) and cotton (or rayon). If you are dyeing only one fiber, it's much better to use a higher-quality, less-expensive dye that doesn't require the commercial dye fixative. Cotton and rayon should be dyed with fiber-reactive dyes; wool and nylon should be dyed with higher quality, more washfast types of acid dye. (Incidentally, higher-quality dyes are nearly always much less expensive, per pound of fabric dyed, than Rit all-purpose dyes.) Clothes that contain both nylon and cotton are not all that common. Cotton-polyester blends are very common, of course, but Rit dye doesn't work on polyester at all, so it's no use for those blends.
The time to use Rit Dye Fixative, or any other cationic dye fixative such as Retayne or Dharma Dye Fixative, is when you don't have a choice of dye type. When you have purchased commercially-dyed fabric or clothing, and found that it's not as washfast as you need it to be, that's the best time to use Retayne. If you've already dyed something with Rit because you have only just now learned that you should have gotten a better dye, then you should use Retayne. If you're about to dye cotton, and haven't started the dyeing process yet, though, you still have time to choose a better dye that will last longer than any of these dye fixatives can, and without the side effects. Rit Dye Fixative and similar products tend to make dye fade more rapidly when exposed to sunlight.
Now, suppose that you have a good reason for using all-purpose dye, such as dyeing a washable garment that contains both nylon and cotton. I still wouldn't want to mix the cationic dye fixative directly into the dyebath. Here's why:
The Rit dye particles (a mixture of acid leveling dye and direct dye) are all negatively charged. So is your fabric. The way the cationic dye fixatives work is that they are positively charged. They associate with both the negatively charged dye molecules and the negatively-charged fiber molecules, more-or-less gluing them together.
When you dye with Rit dye, a large proportion of the dye molecules will not go onto the fabric, but instead stay in the water. This, by the way, is the nature of all cotton dyes, both the direct type and the fiber reactive type. Acid dyes tend to exhaust onto a wool fabric, but when you're dyeing cotton, a large portion of the dye remains in the water.
If you add the positively-charged dye fixative directly to the bath full of excess dye, it will stick directly to the dye molecules, as they float around. This means much of the dye fixer will be wasted, so you'll need to use more. It also means that some of the dye-fixer complexes that are floating around will stick to the fiber, when they might not otherwise have done so; what worries me about this is the idea that they will stick primarily to the outer surfaces of the fibers, instead of penetrating within before sticking. Dyeing the outer surfaces of the fibers in a fabric is called ring-dyeing. It seems to me that it's better to get the dye inside the fiber, and then fix only those dye particles into the fiber, rather than encouraging additional dye to fix onto the outside, where it will tend to wear off, or even crock off (rub off when dry), much more quickly. Admittedly, however, I haven't tested this hypothesis. Most of the time, it's so much easier to just use good permanent types of dye to begin with, and forget about all-purpose dye or the commercial fixatives needed to keep it from bleeding so badly in the laundry.
In addition, remember that all-purpose dye contains a lot of dye that needs to be washed down the drain, since it's a mixture of dye types. The direct dye in the mix that is intended to dye the cotton will not work on nylon or wool, and must be rinsed off of it; the acid dye that is intended to work on nylon or wool should be rinsed off of cotton. When you add the cationic dye fixative, it will tend to make all of these dyes stick to everything. The direct dye will be sticking to the wool, and the acid dye to the cotton. As it does so, however, it won't be sticking as well as it might if it also had an innate affinity for the fiber. Better to get the dye to stick as well as it can by normal dyeing mechanisms, washing away the excess, and then use the fixative to improve matters from there.
Interestingly, the dye fixatives themselves vary widely in how much they cost to use. The cheapest one, Retayne, costs less than a tenth as much to use as the most expensive one, Rit Dye Fixative! Check out this Dye Forum post on a comparison of dye fixative costs. It's cheaper still to use a good fiber reactive dye and skip the use of the dye fixative altogether, though.
(Please help support this web site. Thank you.)
Monday, May 21, 2012
What is the purpose of letting the fabric sit for a period of time after dying it before washing. What is the scientific explanation for this step?
Name: Jenny
Country or region: U.S.
Message: Hi,
I am writing a research paper on the chemistry of dyeing and I want to know what is the purpose of letting the fabric sit for a period of time after dying it before washing. What is the scientific explanation for this step? could you give me a source in which I could find out more about the chemistry involved with dyeing?
It's different for different classes of dye, but the time during which the fabric is allowed to sit with the dye is the reaction time. For fiber reactive dyes, such as Procion MX dyes, a covalent bond is formed between the dye molecule and the textile molecule. Depending on temperature, dye concentration, and other factors, it can take some time for all of the dye molecules to react with the textile fiber molecules. While high-water-ratio immersion dyeing is generally complete within one hour, direct dye application methods such as dye painting or tie-dyeing can require several hours or even up to two days for the last of the dye molecules to react. Some of the dye molecules will react immediately, some will take longer, and some of the dye molecules will take longer still.
What's happening is that the dye molecules are bumbling around in solution waiting to get close enough to an activated textile fiber molecule (a cellulose fiber is activated by a high pH, forming a cellulosic anion). The dye molecule and the fiber generally both have negative electrical charges, which makes them inclined to repel each other. Heat increases the speed with which the molecules tumble around, increasing the rate at which they may encounter a suitable partner in order to undergo the reaction.
This reaction time period after applying the dye and any auxiliary chemicals is often referred to as "batching", referring to the batchwise dyeing of fabric as compared to continuous dyeing processes. Obviously, washing out the unattached dye before all of it has had time to react will result in much wasted dye, and unintentionally pale colors. Washing out dye before it has completed reacting can also cause backstaining, as reactive dye in the rinse water can transfer to unwanted sections of a design; waiting until all dye has reacted, either with the fiber or with the water, means than any backstaining lacks permanent chemical bonds and can be washed out with sufficiently hot water, an impossibility when backstaining is due to still-reactive dyes.
For more information on the chemistry of dyeing, please see the following pages on my website:
Also see the following books:
- David G Duff and Roy S Sinclair: Giles's Laboratory Course in Dyeing, Fourth Edition (1989). ISBN 0 901956 49 X. (This is the best of these books for a beginner to start with.)
- John Shore (ed): Cellulosics Dyeing (1995). ISBN 0 901956 68 6. (Lots of great information, including drawings of reaction mechanisms.)
- Alan Johnson (ed): The Theory of Coloration of Textiles, Second Edition (1989, 1995). ISBN 0-901956 48 1. (This book can be awfully hard going, but really gets into the details.)
- Victor B. Ivanov: Reactive Dyes in Biology. (Originally published 1982; English translation 1987.) Harwood Academic Publishers. ISBN 3-7186-0235-0.
- David R. Waring and Geoffrey Hallas (editors): The Chemistry and Application of Dyes (1990). ISBN 0306432781.
Note that, if your local public library does not have these books, they can get them for you for free via the Interlibrary Loan program, if you are able to wait a few weeks.
(Please help support this web
site. Thank you.)
Sunday, May 20, 2012
Can I check this book on the history of tie-dye out of an American library?
Name: Emari
Country or region: United States
Message: Hi, I'm a student on my way to high school and for my end of the year comm.arts quiz we are doing demonstration speeches and mine is on Tie-dye. Now on your website it has a reference on a book...Michael Abotts Indian and Asian Traditional Textiles. My question is, would I be able to check that out in an American library? Thanks.
There are books on the subject that you should be able to check out of the library, but the one you're referring to is not a book at all. What you're asking about was a link to a website, "Michael Abbott's Indian & Asian Traditional Textiles Website," not to a book; the link appeared on my page, "A Few Notes on the History of Tie Dye". The link to the website had broken, because the page was moved. (I will correct that on my site, thanks for bringing it to my attention.) The correct link should be: http://www.jaintextiles.com/india/tie_dye.htm. The original page was copyrighted in 2000 by Michael Abbott. You can learn more about Michael Abbot here.
The claim that I was referencing with that link was that "Indian Bandhani, one traditional form of tie-dyeing, began some 5000 years ago." Mr. Abbott wrote, about bandhani, "The process is believed to have originated in the area some 5000 years ago and seems to have remained in production ever since."
It would be better for you to get a book from the library, as you suggested, if possible. The book Memory on Cloth: Shibori Now , by Yoshiko Wada, should be available from your local public library system. Its ISBN is 477002777X, and it was published in 2002. If your local library branch does not have it, they may be able to order it from another branch in the same library system. Contact them as soon as possible, since it may take several days for them to get it for you. If they cannot get it from another library branch, then they can certainly order it for you by Interlibrary Loan, but books can take two or more weeks to arrive when ordered from another county or state via Interlibrary Loan. ILL is a wonderful resource, but does take extra planning time. You should also go to your local library, and check to see if there are any other books in the general area of 746.66, in the adult non-fiction area of the library, as this is the Dewey Decimal number for information about tie-dyeing. , by Yoshiko Wada, should be available from your local public library system. Its ISBN is 477002777X, and it was published in 2002. If your local library branch does not have it, they may be able to order it from another branch in the same library system. Contact them as soon as possible, since it may take several days for them to get it for you. If they cannot get it from another library branch, then they can certainly order it for you by Interlibrary Loan, but books can take two or more weeks to arrive when ordered from another county or state via Interlibrary Loan. ILL is a wonderful resource, but does take extra planning time. You should also go to your local library, and check to see if there are any other books in the general area of 746.66, in the adult non-fiction area of the library, as this is the Dewey Decimal number for information about tie-dyeing.
In Memory on Cloth: Shibori Now, on page 28, the author writes, "A great profusion of shibori craft is found in the northwestern part of the Indian subcontinent in the Indus River basin, one of the cradles of human civilization, where archeological finds in Mohenjodaro suggest that dyeing was done as early as 4000 BC. The earliest examples of the most pervasive type of Indian shibori, bandhani, can be seen in the sixth- and seventh-century paintings depicting the life of Buddha found on the wall of Cave 1 at Ajanta. Among the courtiers and ladies in the palace of Price Siddhartha, some wear bodices patterned with spots that seem to be bandhani dots, and many wear wraps with flame-striped patterns like those produced by ikat."
Good luck with your demonstration speech.
(Please help support this web
site. Thank you.)
Wednesday, May 16, 2012
Name: Fatma
—ADVERTISEMENTS—
Books about the chemistry of dyeingExtremely useful for understanding many aspects of different classes of dyes. May be ordered directly from the AATCC (The American Association of Textile Chemists and Colorists) or
 
Country or region: Turkey
Message: Hello, first of all thanks for this web page... in my thesis I am working with removing dye (Remazol Yellow RR) with S. cereviaie. I want to ask you the molecular formula of this dye and also other information about it... thanks a lot....
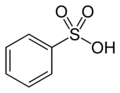
Your Remazol Yellow RR is not listed on my chart of Remazol dyes with their generic names and sources. (See my page, "Vinyl Sulfone Fiber Reactive Dyes".) It does not appear to have a Colour Index name, either, at least as of the most recent information I find on it in a quick search, though what I do find dates from a full decade ago. Sometimes a search with a chemical's CAS number in quotes, in this case "189574-45-6", turns up more information, but that doesn't seem to get us any farther in this case.
You undoubtedly already have the 2007 Demir article [PDF] about decolorization of this dye by a white rot fungus, in which the following properties are given: Structure: Granule
Color: Deep yellow
Odor: Odorless
Melting point: >200°C
Density: 0,48 g/cm3
Aqueous solubility: 100 gl-1 (20°C)
pH: 5-6 (10 gl-1, 20°C)
Flash point: Not determined
Kindling point: >300°C Not much of that is very useful, though, and the density is likely to vary from one batch of the dye powder to another. You should also have the Material Safety Data Sheet from Dystar which was the original source of this information. Various online US tariff documents, however, reveal the full chemical name:
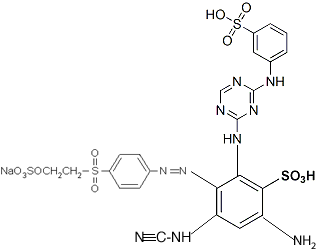
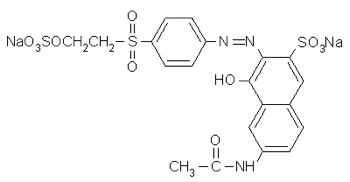
Benzenesulfonic acid, 2-amino-4-(cyanoamino)-6-[(3-sulfophenyl)amino]-1,3,5-triazin-2-yl]amino]-5-[[4-[[2-(sulfoxy)ethyl]sulfonyl]phenyl]azo]-, lithium sodium salt (CAS No. 189574–45–6) ["Remazol Yellow RR Gran"] (source [XLS])
With this name you can figure out how to draw the chemical structure, you can add up the atoms to get the molecular formula, and you can learn a little more about the dye's properties. Unfortunately, the name as given is not quite correct: there are more closing parentheses than opening parentheses, which is confusing. It's good enough to work with, however.
The full chemical names of dyes can be difficult to follow at first, because it's not immediately obvious what parts go where. The secret is to start with the basic dye structure, and figure out where, on that basic form, the numbering indicates that the various sections of the dye molecule should go. Break up the name given above as follows: Benzenesulfonic acid, 2-amino- 4-(cyanoamino)- 6-[(3-sulfophenyl)amino]-1,3,5-triazin-2-yl]amino]- 5-[[4-[[2-(sulfoxy)ethyl]sulfonyl]phenyl]azo]-, lithium sodium salt.
The structure of Remazol Yellow RR is based on benzenesulfonic acid. Benzenesulfonic acid is the following structure: (from Wikipedia). Start by determining which atom, on the benzenesulfonic acid molecule, has which number. Atom number 1 in the benzene ring is the one that has the sulfonic acid on it. Starting in either direction, atom 2 is the one next to it, and so on.
The name indicates that atom number 2 has an amino group, -NH2, on it.
Next, on atom number 4, opposite the sulfonic acid group, is a cyanoamino group, -NH-C≡N. (That's a triple bond between the C and the last N.)
On atom number 5 is [[4-[[2-(sulfooxy) ethyl]sulfonyl] phenyl]azo]-, which contains a beta-sulfatoethylsulfone that is what produces the vinyl sulfone reactive group; it is attached to the benzenesulfonic acid through an azo-bonded phenyl group. The beta-sulfatoethylsulfone is the section that causes the dye to be classed among the Remazol (vinyl sulfone) dyes, and allows it to form a covalent molecular bond to cellulose- and protein-based textile fibers. The vinyl sulfone group itself, -SO2-CH=CH2, is masked on the end with a sulfato group that is be eliminated before the dye reacts with the textile fiber molecule. 
The azo bond, in which two nitrogens are connected to each other by a double bond, is often seen among synthetic dyes, as it is one of the most useful sources of color, especially common among yellows and reds. One frequently hears general statements about the hazards of azo dyes, but in fact it's only a limited subset of azo dyes that are considered dangerous for use. Azo dyes can undergo reductive cleavage at the azo bond to form two amines; the azo dyes banned in Europe are those in which one of the two resulting amines is on a list of 22 known or suspected carcinogens, such as benzidine and 4-chloroaniline. Reductive decolorizing of these dyes occurs at the same time as and by the same process by which a hazardous aromatic amine is regenerated, so decolorizing is not itself a sign that all potentially toxic chemicals have been destroyed.
On atom number 6 is [(3-sulfo phenyl)amino]- 1,3,5-triazin- 2-yl]amino]. Triazine structures are commonly used in reactive dyes; halogen-substituted triazines are particularly useful in forming reactive structures in dye molecules, but that's not what the triazine is being used for in this case. The sulfonate on the end adds to the dye's water-solubility, and presumably this section as a whole does affect the overall color of the molecule.
You can connect the different parts together to give a structure like this: ....though clearly it needs to be redrawn, not forgetting the lithium ion, which was inadvertently omitted in this drawing (instead there is an extra hydrogen in place of it on one of the sulfonates). If you use this structure for anything, you should get someone else to double-check it, in order to be certain I've done it correctly; it's been many years since I did much chemistry (my last degree was in biology).
Compare to other Remazol dyes whose names and structures are both readily available. Here is Remazol yellow 4R, Colour Index reactive yellow 14, whose IUPAC name is sodium 3-chloro-4-[4-[2-methoxy-5-(2-sulfonatooxyethylsulfonyl)phenyl]diazenyl-3-methyl-5-oxo-4H-pyrazol-1-yl]-5-methylbenzenesulfonate:
(from http://www.lookchem.com/REMAZOL-YELLOW-G/)
And compare to Remazol Orange 3R, Colour Index reactive orange 16, whose full name is 2-Naphthalenesulfonic acid, 6-(acetylamino)- 4-hydroxy- 3-((4-((2-(sulfooxy)ethyl) sulfonyl)phenyl)azo)-, disodium salt: With practice you can gather quite a lot of information from the full chemical name of a dye.
(Please help support this web
site. Thank you.)
Monday, May 14, 2012
Can I dye a formal nylon lace, viscose, and cotton dress pale pink?
Name: Kathy
—ADVERTISEMENTS—

Country or region: USA
Message: I just bought an ivory colored (only color that was available) dress knowing I would have to dye it for a wedding. The outer dress has lace which is 60% viscose and 40% nylon, gores in the skirt are 100% nylon, and a separate slip/lining 100% cotton. Is it possible to dye a pale pink? If so, what kind of dye, what temp should the water be, and can it be done in a machine? Please help with this as it's my daughter's wedding and I can't afford to buy anything else or to have it done by a professional.
I think you should return the dress to the store, and somehow find another dress to buy that you won't need to dye. This dress does not sound as though it's washable; if it's not washable, then you won't be able to dye it without ruining it.
It's always disturbing to hear of someone planning to dye a garment they can't afford to replace. Dyeing your dress will probably work, if it is washable—not if it's marked "dry clean only"!—but there's always a chance that it won't work. Dyeing anything that's not sold for the specific purpose of dyeing is a little risky. You always need to have an alternative if you don't like the results.
That said, if you decide to try dyeing the dress anyway, the first thing you must do is wash it. If you can't wash it, you can't dye it, period. If parts of the dress shrink, while others do not, the shape of the dress will be ruined, and you'll have no choice but to find another dress then. (See if there's anything acceptable at a thrift store or consignment store.) Use the hottest water you think the dress will be able to survive, ideally 140°F, because hot water works better to remove invisible stains and finishes, and nylon requires hot water for dyeing. The scary part is that both the cotton and the rayon (viscose) portions of the dress are likely to shrink, while the nylon stays the original size. This is the riskiest step. You can easily ruin the dress at this point, if the manufacturer didn't intend for it to be washed.
Look carefully at the stitching on the dress, at the seams as well as any top-stitching, The thread used to sew a ready-made dress together is always made of polyester, unless the dress is sold as PFD ("Prepared For Dyeing"). That means that it will not take the dye that works on other fibers. The one dye that will work on polyester is unsuitable for a non-polyester dress, since the amount of boiling required for it can cause more damage to the dress. Will the dress look okay once you've dyed the fabric and the lace, if the thread remains white? It probably won't be too bad if you chose a pale color to dye the dress.
If you wash the dress and find that it survived without shrinkage or other damage, then you can consider dyeing it. Viscose rayon can be dyed with the same dyes as cotton, but not with the acid dyes that work on nylon; nylon can be dyed with acid dyes or disperse dyes, but not with the reactive dyes or direct dyes used on cotton and rayon. This is the one situation for which an all-purpose dye is ideal, since it is a mixture of dyes that work on cotton with dyes that work on nylon.
All-purpose dye is inferior for most purposes. The dye tends to bleed in the laundry, so anything dyed with all-purpose dye must be hand-washed separately, and it fades quickly, compared to other dyes. However, neither of those problems will matter for you if you're planning to wear this dress only once, or only a few times. All-purpose dye will not dye polyester, but it will dye both cotton and nylon, as well as rayon (viscose), so it's the best choice for your combination of fibers. Unfortunately, there can be no guarantee that the different fabrics will take the same amount of dye. You may end up with dark pink nylon lace along with pale pink rayon, or the other way around.
There are several different brands of all-purpose dyes. In the US, the easiest brands to find are Rit and Tintex. Some people say the colors in the Tintex line are better. The most common brand of all-purpose dye in the US is Rit, which can often be found in all sorts of stores, even grocery stores or pharmacies. However, grocery stores and pharmacies are likely to have only a limited selection of colors. A fabric store or a crafts store is more likely to have the color you're looking for. It's also possible to use Jacquard acid dye, to color the nylon, and also use Jacquard iDye or Procion dyes, to color the rayon and the cotton, but that's more complicated. Rit all-purpose dye does come in a pink color, if the store you go to has not run out of it. If you can't find pink, you can use a much smaller amount of red dye in order to get pink, using only one-tenth as much dye as the package recommends for dyeing red.
It's difficult to get the color smooth and even unless you use a large container and do a lot of stirring. The easiest way to accomplish this is to dye in the washing machine. For a pale pink, use less dye powder (or liquid dye) than you would use for an intense color. Normally, to get the colored pictured on the package, you would have to use two boxes of the powdered dye, or one bottle of the liquid dye, for each pound of fabric; all-purpose dye does not go very far, because what's in the package is mostly salt and detergent, with only half a teaspoon or so of actual dye included in the mixture in an entire box of dye. In your case, you might want to use only a half a box (or a quarter of a bottle) per pound of fabric (weigh your dress while it's dry to see how many pounds it weighs), to be sure your final color is pale and subtle. You can always repeat your dyeing with more dye if it comes out too light the first time.
To use the dye, use hot water to fill a top-loading washing machine to the lowest setting that will allow enough water for your fabric to move freely. All-purpose dye works best in very hot water, as hot as you can get in your machine, ideally at least 140°F. Dissolve your dye powder in two cups of water in a jar that you will never use again for food, then pour it into the water, taking care to avoid splashing. To help the nylon bond to the dye, you can add one cup of white vinegar per pound of fabric (vinegar will neither help nor hurt the dyeing of the rayon and the cotton). Soak your dress with water, in a sink or bucket, to get it completely wet, then add it to the washing machine and let it agitate on a delicate setting. When the washing machine's timer for the setting gets near the end, probably after about ten minutes, reset the timer to the beginning, so that the water doesn't drain out; keep resetting the timer for at least half an hour. After completing half an hour of dyeing, run the washing machine through one cycle with just cool water to rinse, then repeat with your usual laundry detergent, to wash out excess unattached dye. Afterwards, wash out any visible splashes of dye above the waterline of your washing machine, and wash a load of rags or towels with bleach, to be sure all dye is removed so that it will not stain future loads.
Dyeing with all-purpose dye will probably work well enough for your dress, if it survives the initial washing. I'm afraid that washing it in hot water may ruin it, though. I can't recommend dyeing anything that you can't afford to risk ruining. Good luck.
Sunday, May 13, 2012
Name: Gene
Country or region: U.S
Message: Hello Paula,
I'm thinking of purchasing disperse dye for polyester from prochemicalanddye.com and I was wondering if you have any experience with their disperse dyes and have used it with polyester? I've always believed that to successfully dye polyester is impossible and to have the dye stay on the fabric or be washfast beyond impossible, from my experience. I've been simply trying to tint a 100% polyester fabric a pink and it's become a very difficult task. I've lost all hope in trying to dye and keep the dye on the fabric, and find a dye that will do the job and a company that won't give me issues while ordering said dye. Hopefully you can give me some advice from your years of wisdom in this area.
What kind of dye have you been using? It's no wonder you've had no success if you've only been using dyes intended for cotton and wool until now.
Successfully dyeing polyester is impossible only if you're using the wrong type of dyes. It's not difficult with the right type of dye. Polyester is so different from natural fibers such as cotton or wool that it's pointless to try to dye it with dyes that work on those fibers, but it is easily dyed with disperse dyes, as long as you use water that is hot enough (that is, boiling the dye with the polyester for half an hour or more, not merely using hot tap water), and assuming that the polyester fabric has not been treated with a surface finish that repels dyes. Disperse dye works very well on polyester. Of course, it doesn't work at all well on natural fibers. It's always absolutely essential to match your dye type to the fiber content of your fabric.
The disperse dyes that PRO Chemical & Dye sells will make your dyeing project easy. You will need a cooking pot, one that you don't plan to reuse for food, to boil your polyester in with the disperse dye. This dyeing pot should be made of stainless steel, or else coated with enamel; aluminum is not recommended because it reacts with the acidic ingredients used to protect the dye during boiling.
For dyeing polyester an intense dark color, it is important to also use a smelly and somewhat toxic chemical called a dye carrier or color intensifier; however, since pink is a pale shade of red, you should be able to get by without using the dye carrier additive. That's what I would choose to do. I don't like to use the dye carrier chemical indoors.
It's easy to redye something when you haven't dyed it dark enough. It's far more difficult to remove dye once you have applied it. This means that I would recommend that you aim for a very pale color first, and see what happens. First try using a small amount of dye, in addition to skipping the use of the color intensifier. If, after boiling your polyester with a small amount of red disperse dye, you find that the color is not a bright enough pink, then you can simply add more dye powder, and boil some more.
For any large dyeing project, or any project in which precise results are critical, you should start by running a small-scale test first. Weigh the total amount of fabric that you want to dye. Let's say that your fabric weighs 1000 grams, or 2.2 pounds. You can snip out a test piece that weighs just 10 grams, using a scale that can measure small quantities, like a postal scale does, and then, since your test piece weighs only one-one-hundredth as much as the total, make a miniature dyebath containing 1/100 as much dye powder as you plan to use for the large piece, and 1/100 as much water and any other added chemical. Follow the recipe supplied with your dye, using the same total amount of time boiling. By doing this, you can determine exactly how much of your dye you need to use to get the color you want.
On the other hand, for a reasonably small project in which you can always buy more fabric if it doesn't come out right the first time, you could try plunging right in without doing a test, knowing that the results may be somewhat unpredictable. This can work fine if you don't have to match a precise shade. When precise results are not critical, you can measure your dye by the spoonful, instead of having to weigh out a specific number of grams of dye powder.
When you look at the dyes ProChem sells, you can see that there is no pink dye. Not a problem at all, because pink is simply a dilute red. You can use less dye powder to get the paler color. My rule of thumb for pink is to start with one-tenth as much dye powder as would be required for a bright red. For their D360 Bright Red PROsperse dye, ProChem says to use 1.5% of the weight of the fabric (along with Dye Carrier NSC) to get a bright red hue. That means that, for each pound of fabric (weighed while dry), that is, 454 grams of fabric, you would need about 6.8 grams of dye powder, or, they say, approximately two and a quarter level teaspoons. (One standard teaspoon should contain 5 milliliters, though some kitchen measuring teaspoons can be significantly smaller or larger than this.) If you start by using one-tenth as much dye powder as this, you would need only a little less than one-quarter teaspoon of dye powder, for your first try. That's the amount I would start with if I were using the Dye Carrier NSC in the dyebath. Since colors always come out paler without the use of the Dye Carrier NSC, you might be able to use somewhat more than this without it, but it is impossible, without testing, to predict how MUCH less. That means it's best to start with the least amount you think you might need, and then use more later if the results turn out to be too pale.
For step-by-step instructions, see ProChem's page, "Dyeing Polyester using PROsperse Disperse Dyes" [it's a PDF document]. Carefully read through the directions to see what materials you will need: the disperse dye itself, but also, if your water is at all hard the water softener sodium hexametaphosphate, which ProChem sells under the brand name "Metaphos" (or you can use distilled water instead). You can order citric acid from ProChem, or you can use ordinary white distilled vinegar from the grocery store. Synthrapol is a good detergent to use when dyeing. Soda ash (also known as Dye Activator) can be ordered from ProChem or purchased as sodium carbonate at a swimming pool supply store or hardware store.
Most importantly, you will need a large pot for dyeing in, on the stovetop. You cannot dye polyester in the washing machine, because your water needs to be boiling, not just hot. You will need your dyeing pot pot to be large enough for your fabric to move in freely when you stir it in the water. If the fabric does not move freely enough when you dye it, you will get unevenly color results, lighter in some regions and darker in others, a little like tie-dyeing. If it's important to you to get a single smooth solid color, then you should have plenty of water. A thirty-three quart dyeing pot (a common size for enameled canning pots) is big enough for five gallons of water, which is sufficient for dyeing two pounds of fabric (dry weight). On the other hand, if you don't mind some variation in color intensity, then you can fit more fabric into the same size of pot, as long as there is enough room in the dyebath for the dye and water to penetrate all of the fabric easily.
You will see that dyeing polyester is completely different when you use polyester dye. Cotton dye, as you've seen, simply does not work on any polyester, but polyester dye will.
You asked about my experiences with ordering from PRO Chemical & Dye. I have ordered many dyes from them over the years, and they have consistently been exactly what I ordered and of high quality. As it happens, I think that all of the disperse dyes I've used have come from other sources, but many people I know and trust have done well with ProChem's disperse dyes. It's a good place to order your dyes from.
(Please help support this web site. Thank you.)
Friday, May 11, 2012
How to dye a wool blend coat with Rit All-Purpose dye
Name: Tessa
—ADVERTISEMENTS—


Lanaset Dyes
 Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Buy from Paradise Fibers


Washfast Acid dyes
at Paradise Fibers
 Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!
Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!


  Columbian Home 33-Quart Jar Canner and Rack
Columbian Home 33-Quart Jar Canner and Rack An enameled canner works well as a dyeing pot. Avoid aluminum pots for dyeing, because they react with the vinegar. Do not reuse a pot for cooking food after dyeing in it with Rit or other dyes.
An enameled canner works well as a dyeing pot. Avoid aluminum pots for dyeing, because they react with the vinegar. Do not reuse a pot for cooking food after dyeing in it with Rit or other dyes.
 
Country or region: California
Message: I searched all over your site, and got very good information, but just wanted to make sure I'm doing this with the certainty it won't be a disaster. I have a peacoat, that is 60% wool and 40% polyester, and I would like to dye it dark green, and I have purchased 3 bottles of the green Rit dye. I have used Rit before, but on denim, which I can wash after with no worry, but with this coat, I am not sure how to do the whole process.
I have been looking on the web for ages trying to find information on how to do it, and your site is the closest I have ever come to it.
Please Help me!
There can be no certainty that it won't be a disaster. There are a lot of potential problems:
- Your wool coat might shrink or felt.
- The polyester will not take any of the dye, so the color will only be 60% as dark as you would get if you dyed 100% wool. (Don't even try polyester dyes on polyester-wool blends, because they require much more boiling.)
- Rit All-Purpose dye sometimes produces unexpected colors; it's less reliable than buying a good acid dye for wool.
- The coat might have invisible stains that will resist dye, resulting in one or more random undyed splotches.
- It works much better to use a large cooking pot to heat your wool in. If your cooking pot is not large enough for the coat to move in freely when you stir it, you should expect uneven results, a little like tie-dyeing.
- You probably don't want to dye wool in the washing machine, because the agitation is likely to cause shrinkage. Use the washing machine only if it has a extra-delicate "hand-wash" setting that will allow you to do so without much agitation.
- You should not use an aluminum cooking pot, since aluminum reacts badly with vinegar and other acids.
- You should not use a cooking pot that you will reuse for food, because Rit and other textile dyes are not safe to use on food preparation equipment.
- Dark green dye will color your coat green only if the coat is white to begin with, or if it is light blue, green, or yellow; dye cannot cover up dark colors.
If you decide to go ahead with this project, in spite of the risks, you must first wash the coat thoroughly. Is the coat washable? Try washing it, and see if it survives. Avoid agitation and sudden temperature changes, which are likely to cause shrinkage or damage to the wool. You need to gradually increase the temperature of your dyebath in order to reach the temperature at which the dye will bond to the fiber.
I recommend that you try this only on a coat that you're not interested in wearing again in its current undyed state. It's always possible that you will ruin your coat when you try to dye it.
Rit says that, for dark colors such as Dark Green, you should use one bottle of liquid Rit dye per pound of fabric. (That's twice as much as is required for lighter colors.) For each pound of wool fabric, use one bottle of liquid Rit dye (use more for a darker color), three gallons of water, and one cup of white vinegar; wait to add the vinegar to your dyebath until five minutes or more after combining the fabric with the dye and water. The vinegar helps wool to accept the dye, and it helps to protect the wool from becoming damaged and rough due to the high pH of the detergent ingredients in the Rit dye as packaged.
If your coat is heavy, you're unlikely to find a stainless or enamel cooking pot large enough to dye it in. For a four-pound coat, you should allow at least twelve gallons of water (according to Rit's instructions); I'd rather use at least twice that much water, for a solid level color. Here are the steps to follow:
- Prewash the coat. You cannot dye anything that you have not first washed thoroughly. If it's not washable, it's not dyeable.
- Weigh your coat, while it is dry, to decide how much water and dye you will need. (After weighing it, place it into water to soak so that it will get thoroughly wet before you dye it; a single drop of Synthrapol or hand dishwashing liquid will help the water to penetrate the wool.)
- Multiply the number of pounds the wool coat weighs by three, to find the minimum number of gallons of water you will need.
- If this number is less than 8, buy a 33-quart enamel canning pot to use as a dyeing pot; 33 quarts is the largest size of non-aluminum cookware that is easy to find (look at your local hardware store, or look on Amazon). Used cookware is fine as long as there are no rusty spots or scratches inside. You will not reuse this pot for food after dyeing in it.
- If the number of gallons needed is too large for you to be able to find a suitable size of cooking pot, then you will not be able to heat your dyebath. Find a large bucket or new clean plastic trash can that is large enough to hold the required amount of water, allowing three gallons or more for each pound of fabric. You will probably want to place it either inside the bathtub or shower, or outside (though not near a concrete walkway that can stain), since it will be too heavy to lift once you fill it with water. If possible, find some way to insulate the plastic container so that the dyebath will cool less quickly: wrap it in bubble-wrap or other insulation. It is helpful to place an insulating layer under the container so that it does not lose heat where it contacts the ground, but be careful that the container is steady so that it will not tip.
- Measure the amount of water you need. If you will be using the plastic bucket or trash can, you will need to heat some of the water on the stove, or in a tea kettle, to get it hot enough; your hot tap water is probably not hot enough. It is okay to use your good measuring cups or cooking pot for the clean water, as long as you do not allow dye near them. Remember that one gallon is four quarts, or sixteen cups, or four liters. Try to reach 150°F (that's 66°C), since the water will cool some when you put it into the plastic container; your goal is for the water to be 140°F when you add the wool. Caution: water over 120°F will scald skin on contact, but it works much better for dyeing wool.
- Carefully pour the dye into the water, holding the container just above the surface in order to prevent splashing. (Wear waterproof gloves to protect yourself from the dye and hot water!) If you are using powdered dye, dissolve it in a pint jar of water before adding it to the water.
- Using a long-handled spoon that you will not reuse later for food, or a smooth stick that will not snag your wool, stir the dye in the water until it is evenly mixed. Your stirring spoon or stick needs to be long enough to reach the bottom of the container without getting your hand into the water.
- Add your already-wetted coat to the dyebath. Carefully move it around so that the dye can penetrate evenly, and so that no creases remain in the coat to prevent dye from reaching every part evenly. Stir very slowly and carefully; excessive agitation will cause wool to shrink.
- After five minutes, add the vinegar. Use distilled white vinegar. You will need one cup of vinegar for each pound of fabric. The Rit dye company says to also add one tablespoon of laundry detergent for each pound of fabric, to help level the color. If you have the dyer's detergent Synthrapol on hand, use one teaspoon (5 ml) of it, instead of another laundry detergent.
- Continue to gently move the coat in the dyebath for an hour. Remember that dye will appear lighter after the coat is dry, so you will want it to be very dark in color.
- Allow the wool to cool in the dyebath, then drain off the liquid. It is safe to dispose of it down the drain.
- Rinse the coat in warm water, similar in temperature to the temperature of the dyebath, and then wash in cooler water. Avoid sudden changes in temperature, which will tend to cause shrinkage or felting.
- To reduce the fading suffered by all-purpose dyes such as Rit, follow the dyeing procedure by treating the coat with Retayne, Dharma Dye Fixative, or another commercial dye fixative. Otherwise, be careful to launder the dyed item only in cold water, separately from other garments, or dry clean instead.
Good luck.
(Please help support this web site. Thank you.)
Tuesday, May 08, 2012
I have lots of Cushing's old union dye - what can expect if I attempt to dye wool with the union dye and citric acid?
Name: Brenda
—ADVERTISEMENTS—


Lanaset Dyes
 Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Buy from Paradise Fibers

Country or region: USA
Message: I have lots of Cushing's old union dye - what can expect if I attempt to dye wool with the union dye and citric acid?
The acid dye in union dye, which is another name for all-purpose dye, will work on wool. In fact, it might be the exact same dye that's in Cushing acid dye. The direct dye in the union dye mix will mostly wash out; before washing out, it's possible that it will mislead you a little as to the color of your dye, depending on the formula. Keep in mind that, since there is a lot of waste in the form of the direct dye, you will need to use a much larger amount of dye powder than if you use pure acid dye. Don't measure the union dye by weight or by volume, but instead by the package; unless the writing on the package indicates otherwise, you can assume that one package is supposed to dye one pound of fiber (weighed when dry). Use half a package for half a pound of wool, etc.
I don't know how much acid (if any) may be included in the formula already; there isn't enough acid included for best results in dyeing wool in the Rit or Tintex brands of all-purpose dye, so it's probably a good idea for you to add it. The directions for using Cushing's Acid Dyes [PDF] suggest using one cup of vinegar per pound of fiber. That's similar to using about twenty milliliters (four teaspoons) of citric acid.
The acid dye in all-purpose mixtures such as Cushing's old union dye, like the dye used in Cushing's acid dye, is the type of dye known as strong acid dye, or as acid leveling dye. (See "Leveling Acid Dyes".) It has lower washfastness than other types of acid dye, but is very good at leveling (producing a single solid color). It requires a lower pH than other types of acid dye, so it needs more vinegar or citric acid.
Is there any sort of date anywhere on the package? It looks like Cushing's switch from making union dye to making separate acid and direct dyes may have occurred in 1997 or shortly before, because there's an W. Cushing advertisement in a 1997 volume of the Surface Design Journal that describes the acid dyes and direct dyes as "NEW!". It's important to have some idea of how old your dyes are, because all-purpose dyes packaged before around 1980 are likely to contain benzidine-based dyes, which are known carcinogens and therefore much more dangerous to work with than most other dyes. (See, for example, my blog post about Old Putnam Dyes and another post about benzidine in dyes.) All-purpose dyes sold in the late 1980s and in the 1990s are probably safe, though you should always take safety precautions with any dye, just in case. Wear waterproof gloves, don't use the same pot for dyeing as for cooking, and avoid breathing dye powder.
When you run out of your Cushing dyes, you will find that you can order other types of acid dyes much more economically; these other dyes also have other desirable properties, such as being more resistant to fading when washed. One 1/3-ounce package of Cushing Perfection acid dye, which costs about three dollars, will color one pound of wool. (Use more dye for darker colors, less dye for paler colors.) The cost for enough Lanaset dyes to color one pound of wool is less than a dollar, on average (some colors cost more), even though Lanaset dyes are considered expensive and are extremely high in quality. ProChem's Washfast Acid Dyes cost a little more than half as much as Lanaset dyes, per pound of fiber, but most of them are more washfast than the type of acid dye found in the Cushing dyes. (See the chart I posted in the Dye Forum, "Comparison of Dye Costs".)
(Please help support this web site. Thank you.)
Friday, May 04, 2012
Can I mix Retayne and citric acid in the same bath, so I can use both Cushing acid dye and "ordinary" Cushing dye?
Name: Brenda
—ADVERTISEMENTS—


Lanaset Dyes
 Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Lanaset Dyes are among the very best dyes for hand-dyeing wool, silk, angora, mohair, and most nylons. You will also need: citric acid, sodium acetate, Glauber salt, Albegal SET, and Synthrapol.
Buy from Paradise Fibers

Country or region: USA
Message: I'm using Cushing's dyes to dye wool roving. I have some of their acid dyes, and some of their "ordinary" type dyes. Can I mix Retayne and citric acid in the same bath, so I can use both types of dye? Thank you.
What are the "ordinary" type dyes that you have? W. Cushing makes acid dyes for wool and other protein fibers, and direct dyes for cotton and other cellulose fibers. However, they used to make an all-purpose dye, or union dye, that was a mixture of both the acid dyes and the direct dyes, which they have since discontinued because it makes more sense to choose the right type of dye for your fiber. So, are your "ordinary" Cushing dyes their current direct dyes for cotton, or are they some really old packages of their all-purpose dye? (Acid dyes, direct dyes, and all-purpose dyes stay good for a long time after purchase.)
While it might be possible to make a direct dye work better on wool by mixing it with a cationic dye fixative like Retayne, I don't like the idea at all. It's better to stick to acid dyes when you are dyeing wool. Some direct dyes will function as acid dyes, given the right dyeing conditions, but they're likely not to be quite as good as acid dyes for that purpose. You can be more sure that properly fixed acid dyes will perform predictably on wool, instead of crocking, bleeding, or fading. Dyeing is enough trouble that it's worth using good materials, so that you don't find out later that all of your effort was wasted on an inferior result.
Another important point is that Retayne is not entirely innocuous. (See my page on Retayne, at "Commercial Dye Fixatives".) Cationic dye fixatives like Retayne tend to reduce the lightfastness of dyes. This means that the dyed roving will be more likely to fade in the light, as compared to the same roving dyed with and acid dye and no Retayne. Sunlight is the worst for this, but even indoor lighting can cause fading after some time. (See "Lightfastness of Different Types of Dyes".)
Some people also don't like the idea of the tiny quantity of formaldehyde that is found in all of the cationic dye fixatives that are available for hand-dyers to buy in the US. This does not mean that it requires special safety precautions, though. You should always wear waterproof gloves and safety glasses when working with acid dyes or direct dyes, just as you should when working with Retayne.
(Please help support this web site. Thank you.)
Wednesday, May 02, 2012
Can you tie dye Cotton Sateen?
Name: Mark
Country or region: Minnesota
Message: Can you tie dye Cotton Sateen?
Sure, you can tie-dye cotton sateen. Sateen is a very smooth weave. As long as it's made of an easily dyeable fiber such as cotton, you should be fine.
There is one caveat. Cotton sateen that has been treated to be wrinkle-resistant may not take the dye as well as untreated cotton sateen. It's common for cotton sateen sheets to have wrinkle-resistant treatments. You can still dye the wrinkle-resistant ones, but the colors might not be as smooth or as intense. Still highly worth doing, and cotton sateen has a very nice feel. If you ever see something advertised as being stain-resistant, though, don't even try to dye it.
Be sure to pick decent dyes for tie-dyeing. Do not use Rit all-purpose dye, unless you're using only a single color and don't care about how well it survives laundering. What you need is fiber reactive dye, such as Procion MX dye. This is the kind of dye used in good tie-dyeing kits, such as the Jacquard Tie Dye Kit. For a much wider choice of different colors, you can order your dye online or by phone from a good dye supplier such as Colorado Wholesale Dyes, Aljo Mfg, PRO Chemical & Dye, or Dharma Trading Company. (See "Where to Buy Dyes & Dyeing Supplies".)
Weigh your cotton sateen fabric, while it is still dry. This will help you decide how much dye you need to use. A six-pound set of cotton sheets requires approximately the same amount of dye as twelve adult size XL t-shirts, so one large tie-dyeing kit will do, or two or three small ones, depending on the brand of tie-dyeing kit. (See "How much Procion MX dye should I use?".)
Tuesday, May 01, 2012
Is it safe to use Rit Dye Fixative weeks after applying the dye?
Name: Oubli
Country or region: US
Message: It has taken me weeks to find a fixative, so the clothes that I have dyed have been waiting and waiting for it, the instructions for the Rit Dye Fixative say to use it immediately after dying them. I feel like I messed this up, is it safe to use the fixative now? Do I need alternate instructions to use it? Should I wet down the fabric again before splashing the fixative on it?
Your help would be greatly appreciated. Btw, I did look for this issue on the site but couldn't find anything that pertained to my situation, if I overlooked a post regarding this topic please simply point me in the right direction :)
You didn't mess up. There's no problem. The cationic dye fixatives will work at any time. I've used another brand of a similar product, Retayne, on commercially-dyed garments with no idea as to how long it might have been since they were dyed.
The reason why the instructions for Rit Dye Fixative say to use immediately after dyeing is because there's no requirement to wait, and Rit dye, like all brands of all-purpose dye, is pretty appallingly poorly washfast. If you launder a Rit-dyed garment before applying the fixative, the garment could quickly become badly faded, and ruin any other garments you wash it with. This is not an issue if you've been holding the clothes you dyed aside until you could get the fixative.
Don't splash the fixative on your dyed clothes. It's not intended to be applied undiluted. Instead, fill a bucket or washing machine (depending on how many items you have of the identical color) with very hot tap water, 140°F or a little warmer (to allow for cooling). You'll need plenty of water to ensure a smooth application; use a three-gallon or larger bucket for a single shirt. If your tap water isn't hot enough, you can add some water that you've heated on the stove, but there is no need to spoil your cooking pot by applying the fixative in the pot itself, on the stovetop. It's not considered safe to use Rit Dye Fixative in a cooking pot you will be reusing for food.
Sometimes people try Rit Dye Fixative with Rit All-Purpose Dye for tie-dyeing, but this is not a good idea. Rit Dye Fixative doesn't work for tie-dyed garments that have been dyed in multiple colors, which require a completely different kind of dye. Since Rit Dye Fixative cannot be used without immersing your garment in water, it is suitable only for pieces that have been dyed a single solid color. If you try to tie-dye multiple colors with Rit dye and then fix with Rit Dye Fixative, your colors will bleed together. It's important to use fiber reactive dyes, such as the dyes in good tie-dyeing kits, if you want to tie-dye; if you use fiber reactive dyes, you will not need to use a product like Rit Dye Fixative, because the dyes are permanent without it, when applied according to the instructions.
Wear waterproof gloves and safety glasses when working with Rit Dye Fixative, as it does contain some toxic ingredients. Using a measuring cup and a stick or a long-handled spoon that you will never reuse with food, measure out the necessary amount of Rit Dye fixative into your water and stir it in. Use one-quarter cup of Rit Dye Fixative for one t-shirt. For larger quantities of clothing, weigh all of the clothing of one color before you start, while it is still dry; you will need half a cup of Rit Dye Fixative per pound of fabric. After the Rit Dye Fixative is mixed into the water, you can add your clothing, and stir constantly (or agitate the washing machine) for twenty minutes, so that all of the fabric is treated equally. Apply the dye fixative to only one color of garment at a time. Each color of garment should have the commercial dye fixative added separately; apply the fixative to garments together only if you have dyed them together with the exact same dye color. After the twenty minutes of stirring your clothes with the Rit Dye Fixative in very hot water, you can rinse them in cold water, and then launder as usual before wearing.
I don't see any reason why Rit Dye Fixative shouldn't be as good as other cationic dye fixatives, but their instructions are a little worrisome, because they end with advice to "Be sure to wash dyed garments separately in cold water." This is not a good sign. It's certainly true that some dyes work so differently that the fixative won't help them, but those are vat dyes, not the dyes used in Rit dye packets. What particularly bothers me about this is that, to me, the whole point of using a commercial dye fixative is that you should no longer have to wash the garments separately in cold water! It's important to me to be able to wash my clothes together in full-sized washing machine loads, rather than spending my weekends washing each individual item of clothing I own separately. After I use Retayne to fix commercially-dyed clothing, I always wash the treated items in warm water with other colors of clothing; this has never caused a problem. I hope that Rit Dye Fixative performs better than their instructions suggest. I would advise you to test-launder your treated clothing with a clean white piece of fabric to see whether any color transfers in the wash.
Here are the manufacturers' instructions for Rit Dye Fixative:
Directions
Use immediately after dyeing/rinse cycle to provide long lasting color retention. Washing Machine: Fill machine with Hottest water safe for fabric. Select low water level setting. Add 4 tablespoons Rit Dye Fixative for 1/2 - 3/4 lb. of fabric (or 2 yards fabric). Agitate to mix. Add unfolded fabric. Set washer for extended wash cycle (20 minutes). Rinse in cold water and dry. Clean machine immediately with hot water and detergent using complete cycle. Clean lint traps; wipe spilled fixative with cleanser and water. Sink or Stove Top: Add 4 tablespoons Rit Dye Fixative to 3 gallons of hot water for each 1/2 - 3/4 lb of dry fabric. Add fabric; stir constantly for 20 minutes. Rinse in cold water and dry. Clean container with hot water and cleanser. Note: No fixative will completely prevent dyed garments from bleeding. Fixatives will greatly improve washfastness. Be sure to wash dyed garments separately in cold water.
Safety Warnings
Eye and skin irritant. Harmful if swallowed. Avoid contact with eyes and skin. Wash throughly after handling. First Aid: In case of eye contact, immediately flush with plenty of water for 15 minutes. Call physician. In case of skin contact, flush with water. If swallowed, do not induce vomiting. Give large quantities milk or water. Call physician immediately. Keep out of reach of children.
Rit Dye Fixative is much more dilute than other brands of cationic dye fixative; other brands are more concentrated, so much less of them is required. This means that Rit Dye Fixative is surprisingly more expensive than the other brands of cationic dye fixative. While you must use one-half cup (120 ml) of Rit Dye Fixative for each one to one-and-a-half pounds of fabric, you need only two tablespoons (30 ml) of Jacquard iDye Fixative for one pound of dry fabric. Dharma Dye Fixative also requires one ounce, which is two tablespoons per pound, while Retayne requires only two teaspoons (10 ml) per pound (or one teaspoon per yard of fabric).
It is certainly a good idea to use Rit Dye Fixative or another commercial dye fixative, such as Dharma Dye Fixative, Jacquard iDye Fixative, or Retayne, whenever you've used a poor-quality dye that will fade quickly. Since all-purpose dyes, such as Rit, tend to run badly in the wash, a single mistake in sorting your dyed clothes by color before washing could ruin other clothes. These dye fixatives can help solve the problem of low quality dyes that run in the wash. They don't work as well as using a more permanent dye to begin with, though.
What works best is to use a better dye. Fiber reactive dyes are far more permanent and reliable on cotton than any all-purpose dye, such as Rit. The most popular fiber reactive dye, for home dyers, is Procion MX dye. Other brands that contain fiber reactive dye include tie-dye kits such as the Jacquard Tie Dye Kit, and solid color dyes including Dylon Permanent Dye and Tulip Permanent Dye. These dyes are fixed permanently with soda ash, the same chemical that's in washing soda. They bond so permanently to the fabric that you do not need to use a commercial dye fixative, such as Rit Dye Fixative or Retayne. Dyeing is easier when you don't need to buy special fixatives to make up for poor dye performance. I strongly recommend that you use Procion dye for your next dyeing project, assuming your clothes are made of cotton or similar fibers.
(Please help support this web site. Thank you.)
|
|
 Message: I am trying to dye small areas of a riding vest I wear on my bike. The areas are currently red and I want to dye them like an OD Green (dark green). I looked up to see if I could find out what kind of fabrics it is, the only thing I seemed to find was that it may be Tri-Tex Fabric and Mesh. I have the one that is black and red.
Message: I am trying to dye small areas of a riding vest I wear on my bike. The areas are currently red and I want to dye them like an OD Green (dark green). I looked up to see if I could find out what kind of fabrics it is, the only thing I seemed to find was that it may be Tri-Tex Fabric and Mesh. I have the one that is black and red. 






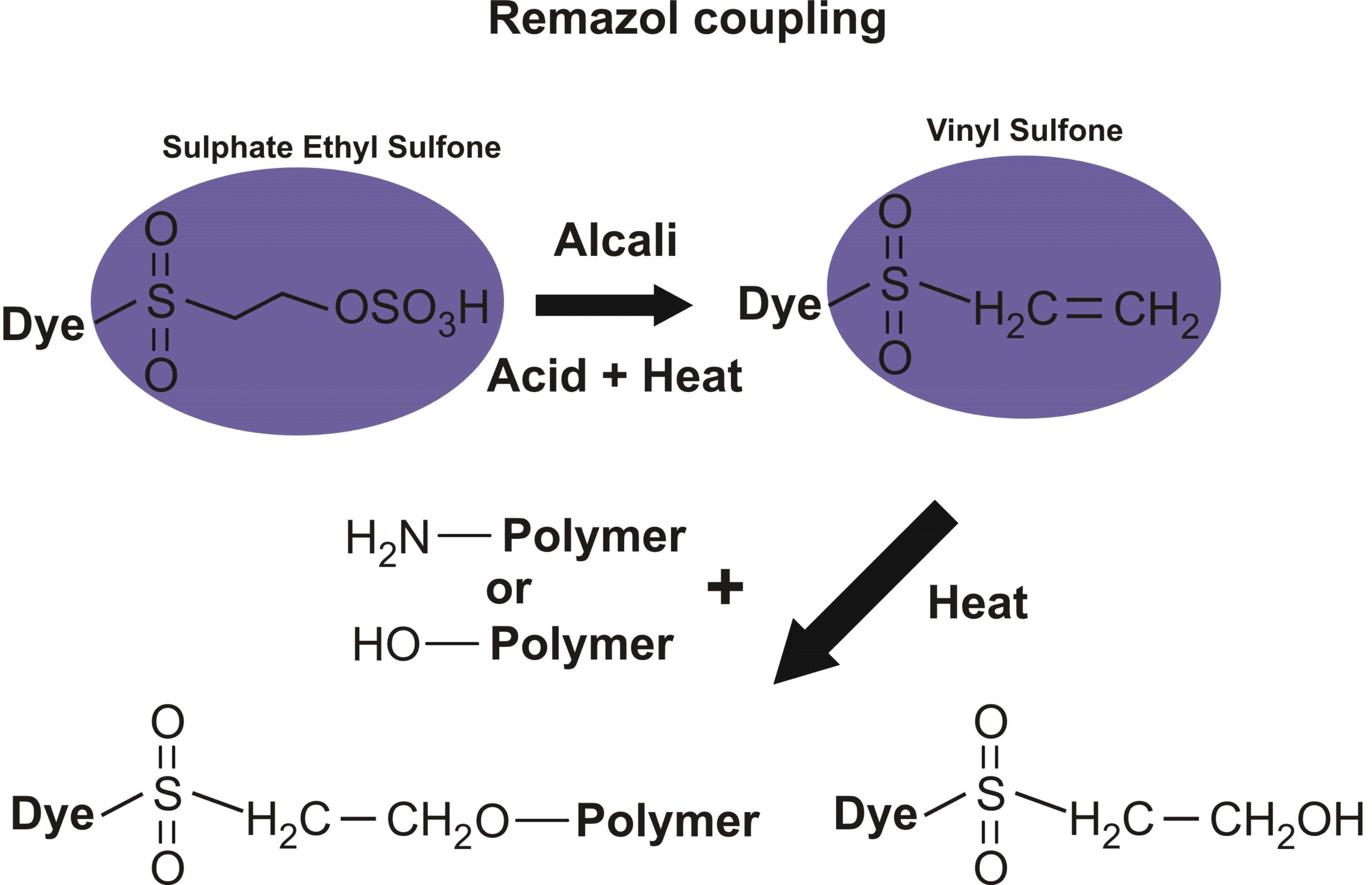
 (The illustrations shown above, with the chromophore shown in yellow or blue and the reactive group shown in violet, were drawn by Dr. Francois Denis, who kindly gave permission for me to use them for educational purposes.)
(The illustrations shown above, with the chromophore shown in yellow or blue and the reactive group shown in violet, were drawn by Dr. Francois Denis, who kindly gave permission for me to use them for educational purposes.)