I am trying to find Saxon Blue indigo liquid extract, as Trudy Van Stralen describes in her book Indigo Madder and Marigold. Do you use or know of a source for this liquid?
Name: Sheila
Message: Wonderful site! I am trying to find Saxon Blue indigo liquid extract, as Trudy Van Stralen describes in her book Indigo Madder & Marigold. Do you use or know of a source for this liquid?
What an interesting question! I do not have Van Stralen's book, but I do have Jim Liles' excellent Art and Craft of Natural Dyeing, which explains that Saxon Blue is indigo that has been dissolved by reacting it with concentrated sulfuric acid, creating an acid dye from what began as a vat dye.
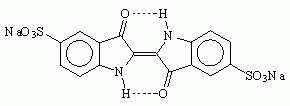
Another name for Saxon blue is Indigo Carmine. Now I have already heard about Indigo Carmine. It is a food coloring, US FD&C Blue No. 2, and is labeled E132 in Europe. Unfortunately, though it is very easy to find large quantities of FD&C blue 1 for sale as a food coloring, FD&C blue 2 seems to be much harder to come by. Interestingly, it is used medicinally in a test of kidney function. If you find a medical source, it will be vastly more expensive than a food coloring source. Another use of indigo carmine is as a pH indicator; it is blue at a pH of 11.6, but yellow at a pH of 13.0. One possible source of indigo carmine, sold for use as a biological stain, is Sargent Welch Chemistry, which sells a tiny 5-gram quantity for $8.50. Try Ward's Natural Science.
Saxon blue was discovered around 1740, according to Liles. He says that it dyes wool and silk without any need for a mordant, producing a striking greenish blue color, quit different from that of vatted indigo. It is not very lightfast, and fabrics dyed with it should not be subjected to strong sunlight. Using alum as a mordant might increase lightfastness and washfastness; Liles is not sure on this point.
Liles' recipe for making your own Saxon blue from indigo is as follows:
1. Into one pound of concentrated sulfuric acid (9 fluid
ounces), stir in slowly and by degrees 3 ounces of best
quality natural indigo or 1.5 ounces (8.5 level
tablespoons) of synthetic indigo. Make sure that the indigo
is very well ground before adding it to the acid (this
applies, primarily, to natural indigo). This should be done
in a strong heat-resistant glass vessel. Use a glass rod
for stirring.
2. Stir the mixture several times during the next few hours
and keep the mixture at about 100°F, if possible.
3. The next day, add gradually, stirring in slowly, about 1
teaspoon of chalk (optional).
4. Stir again the next day. At this point the preparation
is ready to use. Bottle the extract tightly and it will
keep at least a year or two.
5. [Liles wrote] I prefer to keep the product in a rather wide-mouthed
bottle with a good, tight sealing lid so that the material
may be removed by the spoonful.
Note that concentrated sulfuric acid is a highly caustic and dangerous chemical, best used only by those with training in working safely with chemicals. If I were to follow this recipe, I would wear a heavy coated apron, heavy thick rubber gloves, and a full plastic face shield, in case the sulfuric acid splatters. I would not be concerned about carcinogenicity or long-term toxic effects, though, only about the strong acid. None of the ingredients in the above recipeare poisonous once they have been diluted with large enough amounts of water to increase the pH to something reasonably close to neutral, but the concentrated sulfuric acid is such as strong acid as to warrant care in handling.
Liles also gives recipes for using Saxon blue to dye silk or wool, and another recipe for using it to dye cotton or linen. The latter, he says, is not very satisfactory and was used only for making greens, by overdyeing with yellow. Apparently the use of Saxon blue on silk or wool is quite satisfactory, however, as long as you do not expose the resulting fabric to bright light or sunlight very much.
(Please help support this web site. Thank you.)
Message: Wonderful site! I am trying to find Saxon Blue indigo liquid extract, as Trudy Van Stralen describes in her book Indigo Madder & Marigold. Do you use or know of a source for this liquid?
What an interesting question! I do not have Van Stralen's book, but I do have Jim Liles' excellent Art and Craft of Natural Dyeing, which explains that Saxon Blue is indigo that has been dissolved by reacting it with concentrated sulfuric acid, creating an acid dye from what began as a vat dye.

Another name for Saxon blue is Indigo Carmine. Now I have already heard about Indigo Carmine. It is a food coloring, US FD&C Blue No. 2, and is labeled E132 in Europe. Unfortunately, though it is very easy to find large quantities of FD&C blue 1 for sale as a food coloring, FD&C blue 2 seems to be much harder to come by. Interestingly, it is used medicinally in a test of kidney function. If you find a medical source, it will be vastly more expensive than a food coloring source. Another use of indigo carmine is as a pH indicator; it is blue at a pH of 11.6, but yellow at a pH of 13.0. One possible source of indigo carmine, sold for use as a biological stain, is Sargent Welch Chemistry, which sells a tiny 5-gram quantity for $8.50. Try Ward's Natural Science.
Saxon blue was discovered around 1740, according to Liles. He says that it dyes wool and silk without any need for a mordant, producing a striking greenish blue color, quit different from that of vatted indigo. It is not very lightfast, and fabrics dyed with it should not be subjected to strong sunlight. Using alum as a mordant might increase lightfastness and washfastness; Liles is not sure on this point.
Liles' recipe for making your own Saxon blue from indigo is as follows:
1. Into one pound of concentrated sulfuric acid (9 fluid
ounces), stir in slowly and by degrees 3 ounces of best
quality natural indigo or 1.5 ounces (8.5 level
tablespoons) of synthetic indigo. Make sure that the indigo
is very well ground before adding it to the acid (this
applies, primarily, to natural indigo). This should be done
in a strong heat-resistant glass vessel. Use a glass rod
for stirring.
2. Stir the mixture several times during the next few hours
and keep the mixture at about 100°F, if possible.
3. The next day, add gradually, stirring in slowly, about 1
teaspoon of chalk (optional).
4. Stir again the next day. At this point the preparation
is ready to use. Bottle the extract tightly and it will
keep at least a year or two.
5. [Liles wrote] I prefer to keep the product in a rather wide-mouthed
bottle with a good, tight sealing lid so that the material
may be removed by the spoonful.
Note that concentrated sulfuric acid is a highly caustic and dangerous chemical, best used only by those with training in working safely with chemicals. If I were to follow this recipe, I would wear a heavy coated apron, heavy thick rubber gloves, and a full plastic face shield, in case the sulfuric acid splatters. I would not be concerned about carcinogenicity or long-term toxic effects, though, only about the strong acid. None of the ingredients in the above recipeare poisonous once they have been diluted with large enough amounts of water to increase the pH to something reasonably close to neutral, but the concentrated sulfuric acid is such as strong acid as to warrant care in handling.
Liles also gives recipes for using Saxon blue to dye silk or wool, and another recipe for using it to dye cotton or linen. The latter, he says, is not very satisfactory and was used only for making greens, by overdyeing with yellow. Apparently the use of Saxon blue on silk or wool is quite satisfactory, however, as long as you do not expose the resulting fabric to bright light or sunlight very much.
(Please help support this web site. Thank you.)
Posted: Tuesday - July 24, 2007 at 11:16 AM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM