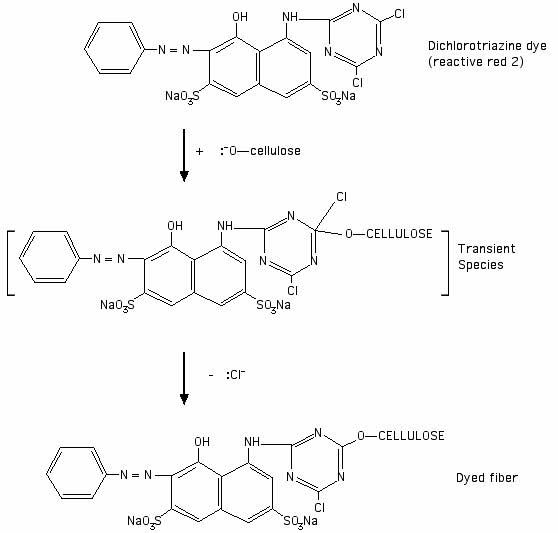
Are the bonds that connect dye and fabric polar covalent or non-polar covalent?
—ADVERTISEMENT—

Linda Knutson's
Synthetic Dyes for Natural Fibers
Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
Burch, Paula. "Are the Bonds That Connect Dye and Fabric Polar Covalent or Non-polar Covalent?"Paula Burch's All About Hand Dyeing. 11 January 2011. <http://www.pburch.net/dyeing/dyeblog/C1845207367/E20110111074828/index.html>(Please help support this web site. Thank you.)
Posted: Tuesday - January 11, 2011 at 07:48 AM All About Hand Dyeing Q&A Previous Next
Follow this blog on twitter here.
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Follow this blog on twitter here.
Calendar
Search
Categories
- about dyes
- batik
- big projects
- business of dyeing
- changing color of clothing
- chemistry of dyeing
- color mixing
- disasters and problems
- discharge (bleach) dyeing
- dye & fiber choice
- dye application
- dye safety
- dyeing acrylic
- dyeing furniture
- dyeing nylon
- dyeing objects
- dyeing polyester
- finding a dyer
- fixing dye
- immersion or vat dyeing
- miscellaneous
- natural dyes
- pigments and fabric paint
- rainbow dyeing
- removing dye
- satin can be any fiber
- schoolwork
- screen prints & dye
- they want to sell us dyes
- tie dye parties
- tie-dyeing, resists, shibori
- washing out after dyeing
- where to get dyes and supplies
Archives
XML/RSS Feed
Statistics
All About Hand Dyeing Q&A: 2223
about dyes: 176
batik: 57
big projects: 12
business of dyeing: 8
changing color of clothing: 357
chemistry of dyeing: 105
color mixing: 53
disasters and problems: 68
discharge (bleach) dyeing : 32
dye & fiber choice: 209
dye application: 72
dye safety: 49
dyeing acrylic: 2
dyeing furniture: 49
dyeing nylon: 46
dyeing objects: 33
dyeing polyester: 125
finding a dyer: 50
fixing dye: 106
immersion or vat dyeing: 20
miscellaneous: 127
natural dyes: 30
pigments and fabric paint: 48
rainbow dyeing: 11
removing dye: 63
satin can be any fiber: 9
schoolwork: 43
screen prints & dye: 12
they want to sell us dyes: 2
tie dye parties: 23
tie-dyeing, resists, shibori: 56
washing out after dyeing: 32
where to get dyes and supplies: 133
– ADVERTISEMENTS –
Tie-dye kits make great gifts!
Total entries in this category:
Published On: Aug 29, 2012 02:49 PM

 Syndicate this site
Syndicate this site