Satin is a weave, not a fiber. Satin can be made out of many different natural or synthetic fibers. This means that it is impossible to tell you how to dye your satin without knowing what fiber your satin is made of. If you have a dress made of polyester satin, the answer will be completely different than if your dress is made of 100% natural silk satin.
To find out for sure what fiber you have, snip a small amount of fabric out of a seam allowance somewhere, where it won't show, and try a burn test. Two excellent resources for interpreting the results of a fiber burn test are Griffin Dyeworks' Burn Test page (or see their PDF) and Ditzy Prints' Fiber Burn Chart. To test for acetate or triacetate, try dissolving a bit of thread from your fabric in some acetone-containing nail polish remover.
If your satin is made from polyester or acetate, you will need to boil it for half an hour or longer with a special synthetic-fiber dye called disperse dye. Polyester will not take any other sort of dye at all; if you try to dye polyester with an all-purpose dye, such as Rit, or a fiber-reactive dye such as Procion, or an acid dye, the dye will just wash out. Acetate may stain with all-purpose or acid dye, but the results will not be good; use disperse dye for acetate. See "Dyeing Polyester with Disperse Dyes".
Satin that is woven from silk is the most luxurious kind. It is also easy to dye.
The easiest way to dye silk satin or silk charmeuse is by using a fiber reactive dye, such as Procion MX dye, along with soda ash as the dye fixative. Unlike most dyes, Procion MX and a few other fiber reactive dyes have the advantage of being usable at room temperature, with no heat setting required. This is the exact same method as is used to dye cotton or rayon with Procion MX dyes; in fact, a good tie-dye kit will work very well. The luster of silk will be slightly reduced, and the softness increased, by the high pH of soda ash; a final rinse containing a little vinegar will partially restore the loss.
If you do not want your silk satin to lose even a little of its luster, you should avoid the use of soda ash, and use an acid, such as vinegar, instead. You can use any sort of acid dye on silk. If you use fiber reactive dyes with an acid instead of soda ash, they too will function as acid dyes; see "Fiber reactive dyes on protein fibers. For good results with acid dyes on silk, you must either use a hot dyebath, up to 185°F (87°C), or allow the dye to dry, wrap the silk in paper, and steam the fabric, in order to enable the dyes to bond to the fiber. The amount of time required for steaming varies from thirty minutes for Procion MX, Procion H, and Remazol dyes, to three hours for the French silk dyes, including Sennelier Tinfix, H. Dupont, and Pebeo Soie dyes.
Nylon satin, if untreated, can be dyed with most acid dyes. Unfortunately, many nylon fabrics are coated with substances that tend to repel dyes, resulting in unsatisfactory dyeing. See "How to dye nylon or polyamide".
Rayon satin will dye beautifully at room temperature with fiber reactive dyes, such as Procion MX dyes. The colors will be more intense than obtained on unmercerized cotton, but otherwise similar. See "How to dye rayon". If you can't find a source of rayon satin locally, you can mail-order it from Dharma Trading Company (see Sources for Dyeing Supplies).
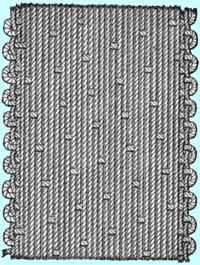 Satin is woven by running the warp thread over four or more of the weft yarns, often varying from one warp thread to the next in how many weft threads are covered at a time so that there is no regular grain. The long warp loops, called floats, can be snagged more easily than those that pass over only one or two filling yarns, so satin is a relatively fragile weave.
Satin is woven by running the warp thread over four or more of the weft yarns, often varying from one warp thread to the next in how many weft threads are covered at a time so that there is no regular grain. The long warp loops, called floats, can be snagged more easily than those that pass over only one or two filling yarns, so satin is a relatively fragile weave.
A satin weave made with cotton fibers can be called satin, but is more commonly called sateen. Cotton sateen is usually less glossy than other satins, because the threads used to weave it are less shiny.
Charmeuse is a satin weave in which the warp yarns are tightly twisted, and the weft yarns crepe or spun twisted yarns. (Source: All About Silk: A Fabric Dictionary & Swatchbook, by Julie Parker, 1991.)
 Back to list of FAQs
Back to list of FAQs
Last updated: February 7, 2009
Page created: February 7, 2009
Downloaded: Sunday, February 22, 2026
All of the pages on this site are copyright ©1998‑2026 Paula E. Burch, Ph.D.