Advertisement
Buy Jacquard Urea
Advertisements
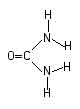 Urea is used in tie dyeing and other forms of direct application of dyes.
It is usually not used in vat dyeing or low water immersion dyeing.
Urea is used in tie dyeing and other forms of direct application of dyes.
It is usually not used in vat dyeing or low water immersion dyeing.
Urea has two purposes: it can make it possible to dissolve more dye in a given volume, for the strongest of colors, and it serves as a humectant, or water-attractor, to help keep fabric damp long enough for the reaction to occur. You can do without urea if your dye solutions are strong enough for your needs without it, and you keep your fabric damp in some other way, such as by covering with plastic.
Too much urea can actually make it harder to dissolve some dyes, according to Craig Turner of Standard Dyes, who says that, if your dye (especially fuchsia, Procion red MX-8B) is not dissolving well, you may need to reduce the amount of urea you are using.
Urea is the same chemical as is found in the urine of mammals, but the urea we buy is not obtained from urine. It is synthesized from natural gas.
No, urea is reasonably safe, compared to many other chemicals. It's a major ingredient in many skin moisturizers, due to its humectant properties. Although you should avoid skin exposure to dyes or soda ash, skin exposure to urea is not a major safety issue. It may be irritating to the skin. Airborne urea, like most powders, is hazardous when inhaled.
Yes, urea will, given enough time, break down to form ammonia. Your dyeing urea should have no particular odor. If it has a strong, unpleasant smell, discard it (you can use it as fertilizer), because you do not want to be using ammonia. Ammonia will increase the pH of solutions to which it is added, and is irritating to the user.
Any dye supplier should be able to sell you nice clean urea.
For buying in bulk, try a local feed store. Ask for "pure shotted urea" if available, or 46-0-0 fertilizer. Some grades of urea are nice and clean, while others are dirty, contaminated with bits of darker material. Look before buying, to see if the urea is clean enough to suit you.
Advertisements
 Back to list of FAQs
Back to list of FAQs
All of the pages on this site are copyright ©1998‑2026 Paula E. Burch, Ph.D.
Page created: Saturday, November 13, 1999
Last updated: April 26, 2008
Downloaded: Sunday, February 22, 2026